Preoperative chemoradiation with capecitabine, irinotecan and cetuximab in rectal cancer: significance of pre-treatment and post-resection RAS mutations
- PMID: 28859058
- PMCID: PMC5672930
- DOI: 10.1038/bjc.2017.294
Preoperative chemoradiation with capecitabine, irinotecan and cetuximab in rectal cancer: significance of pre-treatment and post-resection RAS mutations
Abstract
Background: The influence of EGFR pathway mutations on cetuximab-containing rectal cancer preoperative chemoradiation (CRT) is uncertain.
Methods: In a prospective phase II trial (EXCITE), patients with magnetic resonance imaging (MRI)-defined non-metastatic rectal adenocarinoma threatening/involving the surgical resection plane received pelvic radiotherapy with concurrent capecitabine, irinotecan and cetuximab. Resection was recommended 8 weeks later. The primary endpoint was histopathologically clear (R0) resection margin. Pre-planned retrospective DNA pyrosequencing (PS) and next generation sequencing (NGS) of KRAS, NRAS, PIK3CA and BRAF was performed on the pre-treatment biopsy and resected specimen.
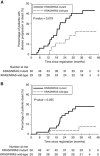
Results: Eighty-two patients were recruited and 76 underwent surgery, with R0 resection in 67 (82%, 90%CI: 73-88%) (four patients with clinical complete response declined surgery). Twenty-four patients (30%) had an excellent clinical or pathological response (ECPR). Using NGS 24 (46%) of 52 matched biopsies/resections were discrepant: ten patients (19%) gained 13 new resection mutations compared to biopsy (12 KRAS, one PIK3CA) and 18 (35%) lost 22 mutations (15 KRAS, 7 PIK3CA). Tumours only ever testing RAS wild-type had significantly greater ECPR than tumours with either biopsy or resection RAS mutations (14/29 [48%] vs 10/51 [20%], P=0.008), with a trend towards increased overall survival (HR 0.23, 95% CI 0.05-1.03, P=0.055).
Conclusions: This regimen was feasible and the primary study endpoint was met. For the first time using pre-operative rectal CRT, emergence of clinically important new resection mutations is described, likely reflecting intratumoural heterogeneity manifesting either as treatment-driven selective clonal expansion or a geographical biopsy sampling miss.
Conflict of interest statement
Outside of the submitted work SG has received research funding from Roche and Pfizer. NW reports grants from Yorkshire Cancer Research, grants from Pathological Society of Great Britain and Ireland, during the conduct of the study; grants from Academy of Medical Sciences, outside the submitted work. DS-M has received research funding from Roche and Sanofi-Aventis. PQ reports personal fees from Amgen, personal fees from Roche, personal fees from Ventana, during the conduct of the study; grants from Yorkshire Cancer Research programme grant, within and outside the submitted work. BS reports personal fees from Roche, personal fees and non-financial support from Sanofi and non-financial support from BMS, outside the submitted work. SB reports grants from Merck, grants from Pfizer Limited, during the conduct of the study. The remaining authors declare no conflict of interest.
Figures
References
-
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354: 567–578. - PubMed
-
- Bosset J-F, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun R-J, Bardet E, Beny A, Ollier J-C, Bolla M, Marchal D, Van Laethem J-L, Klein V, Giralt J, Clavère P, Glanzmann C, Cellier P, Collette L (2014) Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 15: 184–190. - PubMed
-
- Clancy C, Burke JP, Coffey JC (2013) KRAS mutation does not predict the efficacy of neo-adjuvant chemoradiotherapy in rectal cancer: a systematic review and meta-analysis. Surg Oncol 22: 105–111. - PubMed
-
- Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J, Ray R, Bashir N, Bridgewater JA, Geh JI, Cunningham D, Blazeby J, Roy R, Maughan T, Griffiths G (2013) Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol 14: 627–637. - PubMed
-
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351: 337–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

