Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity
- PMID: 28859530
- PMCID: PMC5815295
- DOI: 10.1080/10253890.2017.1369523
Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity
Abstract
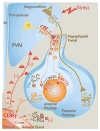
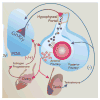
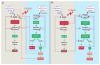
Gonadal hormones play a key role in the establishment, activation, and regulation of the hypothalamic-pituitary-adrenal (HPA) axis. By influencing the response and sensitivity to releasing factors, neurotransmitters, and hormones, gonadal steroids help orchestrate the gain of the HPA axis to fine-tune the levels of stress hormones in the general circulation. From early life to adulthood, gonadal steroids can differentially affect the HPA axis, resulting in sex differences in the responsivity of this axis. The HPA axis influences many physiological functions making an organism's response to changes in the environment appropriate for its reproductive status. Although the acute HPA response to stressors is a beneficial response, constant activation of this circuitry by chronic or traumatic stressful episodes may lead to a dysregulation of the HPA axis and cause pathology. Compared to males, female mice and rats show a more robust HPA axis response, as a result of circulating estradiol levels which elevate stress hormone levels during non-threatening situations, and during and after stressors. Fluctuating levels of gonadal steroids in females across the estrous cycle are a major factor contributing to sex differences in the robustness of HPA activity in females compared to males. Moreover, gonadal steroids may also contribute to epigenetic and organizational influences on the HPA axis even before puberty. Correspondingly, crosstalk between the hypothalamic-pituitary-gonadal (HPG) and HPA axes could lead to abnormalities of stress responses. In humans, a dysregulated stress response is one of the most common symptoms seen across many neuropsychiatric disorders, and as a result, such interactions may exacerbate peripheral pathologies. In this review, we discuss the HPA and HPG axes and review how gonadal steroids interact with the HPA axis to regulate the stress circuitry during all stages in life.
Keywords: Hypothalamic–pituitary–adrenal axis; estrogen; gonadal steroids; hypothalamic–pituitary–gonadal axis; progesterone; sex differences; stress circuitry; testosterone.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
References
-
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, … Maeda K. Involvement of anteroventral periventricular meta-stin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. Journal of Reproduction and Development. 2007;53:367–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
