Predictive value of C-reactive protein in patients treated with sunitinib for metastatic clear cell renal cell carcinoma
- PMID: 28859644
- PMCID: PMC5580299
- DOI: 10.1186/s12894-017-0267-6
Predictive value of C-reactive protein in patients treated with sunitinib for metastatic clear cell renal cell carcinoma
Abstract
Background: Sunitinib has become mainstay first line treatment for patients with metastatic renal clear cell carcinoma (mRCC). Still, useful predictive markers of response are lacking and urgently needed for clinical decision making.
Methods: In the present study we investigated the predictive value of standard serum markers as well as clinical markers, including C-reactive protein (CRP), Neutrophil to Lymphocyte ratio (NLR) and early hypertension (eHTN) in an unselected prospective patient population treated with sunitinib for mRCC. Forty-six patients were enrolled in a prospective single-arm study of predictive markers for sunitinib response. Response rates according to RECIST 1.1 were used as primary end-point. Secondary objectives were to evaluate prognostic value of the candidate markers with regard to progression free survival (PFS) and overall survival (OS). In addition, toxicity rates and quality of life was recorded.
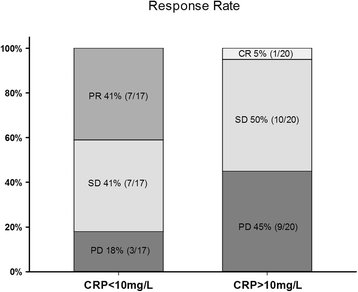
Results: Median PFS and OS was 9.1 months and 15.0 months, respectively. Of 38 patients evaluable for response, 1 patient had complete response (CR), 7 had partial response (PR), 18 had stable disease (SD) and 12 had progressive disease (PD). Normal CRP at baseline was significantly associated with objective response (CR + PR) (p = 0.01). Normal CRP was also significantly associated with improved PFS and OS (Log rank, p = 0.05 and <0.01, respectively). Early hypertension, NLR and IMDC risk score were not significantly associated with response rates or survival.
Conclusion: Baseline CRP was a significant predictive factor of sunitinib response and a prognostic factor of survival. Baseline CRP might be a useful biomarker in the treatment planning of mRCC. Due to the relatively small sample size, our results need to be confirmed in larger studies.
Conflict of interest statement
Ethics approval and consent to participate
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice. The protocol was approved by the Regional Ethics Committee (REK number 080/07) and the Norwegian Medicines Agency. All participating patients provided signed informed consent before enrolment.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590. doi: 10.1200/JCO.2008.20.1293. - DOI - PMC - PubMed
-
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al. Nivolumab versus Everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015; - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

