Synergistic effects of aerobic exercise and cognitive training on cognition, physiological markers, daily function, and quality of life in stroke survivors with cognitive decline: study protocol for a randomized controlled trial
- PMID: 28859664
- PMCID: PMC5579904
- DOI: 10.1186/s13063-017-2153-7
Synergistic effects of aerobic exercise and cognitive training on cognition, physiological markers, daily function, and quality of life in stroke survivors with cognitive decline: study protocol for a randomized controlled trial
Abstract
Background: Aerobic exercise and cognitive training have been effective in improving cognitive functions; however, whether the combination of these two can further enhance cognition and clinical outcomes in stroke survivors with cognitive decline remains unknown. This study aimed to determine the treatment effects of a sequential combination of aerobic exercise and cognitive training on cognitive function and clinical outcomes.
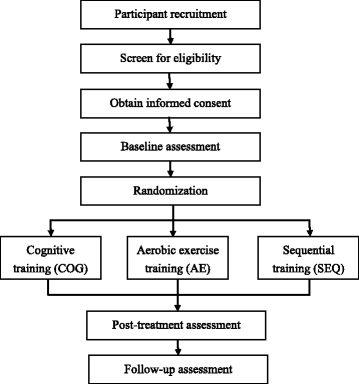
Methods/design: Stroke survivors (n = 75) with cognitive decline will be recruited and randomly assigned to cognitive training, aerobic exercise, and sequential combination of aerobic exercise and cognitive training groups. All participants will receive training for 60 minutes per day, 3 days per week for 12 weeks. The aerobic exercise group will receive stationary bicycle training, the cognitive training group will receive cognitive-based training, and the sequential group will first receive 30 minutes of aerobic exercise, followed by 30 minutes of cognitive training. The outcome measures involve cognitive functions, physiological biomarkers, daily function and quality of life, physical functions, and social participation. Participants will be assessed before and immediately after the interventions, and 6 months after the interventions. Repeated measures of analysis of variance will be used to evaluate the changes in outcome measures at the three assessments.
Discussion: This trial aims to explore the benefits of innovative intervention approaches to improve the cognitive function, physiological markers, daily function, and quality of life in stroke survivors with cognitive decline. The findings will provide evidence to advance post-stroke cognitive rehabilitation.
Trial registration: ClinicalTrials.gov, NCT02550990 . Registered on 6 September 2015.
Keywords: Aerobic exercise; Cognitive rehabilitation; Cognitive training; Sequential training; Stroke.
Conflict of interest statement
Authors’ information
TTY, School of Occupational Therapy, College of Medicine, National Taiwan University, Taipei, Taiwan; CYW and YWH: Department of Occupational Therapy and Graduate Institute of Behavioral Sciences, College of Medicine, Chang Gung University, Taoyuan, Taiwan, Healthy Aging Research Center, Chang Gung University, Taoyuan, Taiwan, Department of Physical Medicine and Rehabilitation, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan; KCC: Department of Neurology, Chang Gung Memorial Hospital, Kaohsiung, Taiwan; LCL: Department of Physical Medicine and Rehabilitation, Cheng Hsin General Hospital, Taipei, Taiwan; JWH: Department of Rehabilitation, Chang Gung Memorial Hospital-Kaohsiung Medical Center, Chang Gung University, College of Medicine, Kaohsiung, Taiwan; KCL: School of Occupational Therapy, College of Medicine, National Taiwan University, Taipei, Taiwan, Division of Occupational Therapy, Department of Physical Medicine and Rehabilitation, National Taiwan University Hospital, Taipei, Taiwan; CHT: Department of Occupational Therapy and Graduate Institute of Behavioral Sciences, College of Medicine, Chang Gung University, Taoyuan, Taiwan; YHL: Department of Occupational Therapy and Graduate Institute of Behavioral Sciences, College of Medicine, Chang Gung University, Taoyuan, Taiwan, Healthy Aging Research Center, Chang Gung University, Taoyuan, Taiwan.
Ethics approval and consent to participate
Ethical approval has been obtained through the centralized ethics committee of Chang Gung Memorial Hospital Human Research Ethics Board (IRB# 104-4892A3). This trial is registered with ClinicalTrials.gov Identifier, NCT02550990. Ethical approval includes agreed processes for obtaining consent from (or on behalf of) all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Jacquin A, Binquet C, Rouaud O, et al. Post-stroke cognitive impairment: high prevalence and determining factors in a cohort of mild stroke. J Alzheimers Dis. 2014;40:1029–38. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical