Multiple therapeutic effect of endothelial progenitor cell regulated by drugs in diabetes and diabetes related disorder
- PMID: 28859673
- PMCID: PMC5580204
- DOI: 10.1186/s12967-017-1280-y
Multiple therapeutic effect of endothelial progenitor cell regulated by drugs in diabetes and diabetes related disorder
Abstract
Background: Reduced levels of endothelial progenitor cells (EPCs) counts have been reported in diabetic mellitus (DM) patients and other diabetes-related disorder. EPCs are a circulating, bone marrow-derived cell population that appears to participate in vasculogenesis, angiogenesis and damage repair. These EPC may revert the damage caused in diabetic condition. We aim to identify several existing drugs and signaling molecule, which could alleviate or improve the diabetes condition via mobilizing and increasing EPC number as well as function.
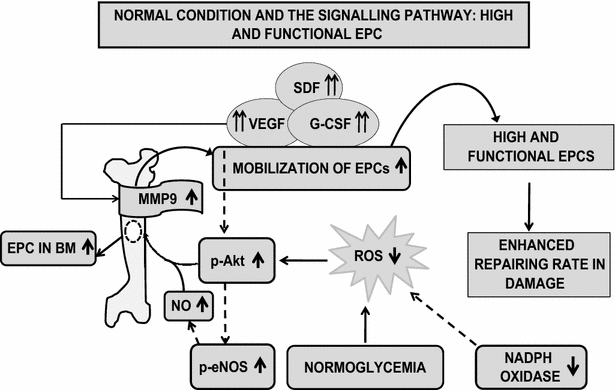
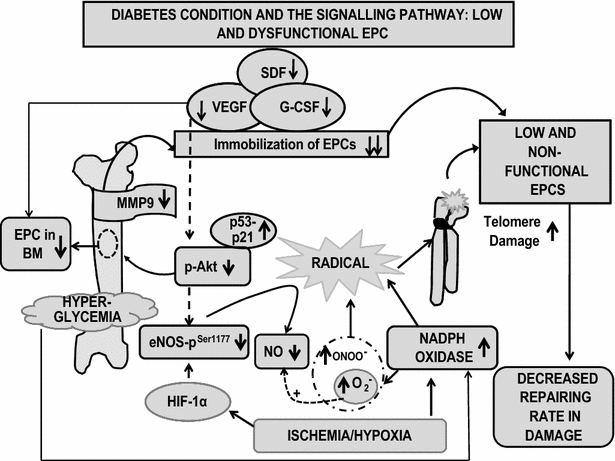
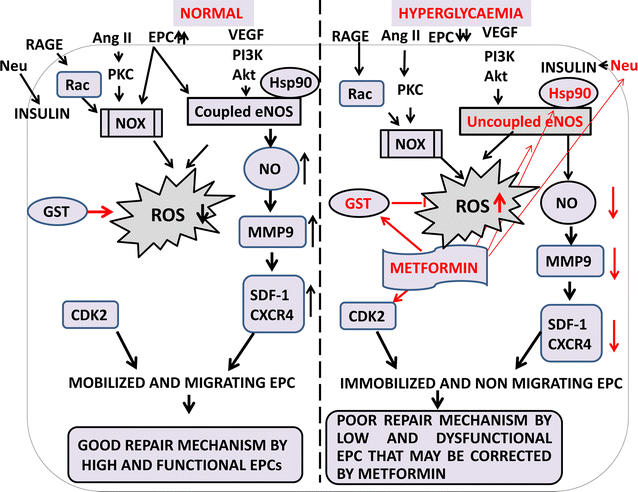
Main body: Accumulated evidence suggests that dysregulation of EPC phenotype and function may be attributed to several signaling molecules and cytokines in DM patients. Hyperglycemia alone, through the overproduction of reactive oxygen species (ROS) via eNOS and NOX, can induce changes in gene expression and cellular behavior in diabetes. Furthermore, reports suggest that EPC telomere shortening via increased oxidative DNA damage may play an important role in the pathogenesis of coronary artery disease in diabetic patients. In this review, different type of EPC derived from different sources has been discussed along with cell-surface marker. The reduced number and immobilized EPC in diabetic condition have been mobilized for the therapeutic purpose via use of existing, and novel drugs have been discussed. Hence, evidence list of all types of drugs that have been reported to target the same pathway which affect EPC number and function in diabetes has been reviewed. Additionally, we highlight that proteins are critical in diabetes via polymorphism and inhibitor studies. Ultimately, a lucid pictorial explanation of diabetic and normal patient signaling pathways of the collected data have been presented in order to understand the complex signaling mystery underlying in the diseased and normal condition.
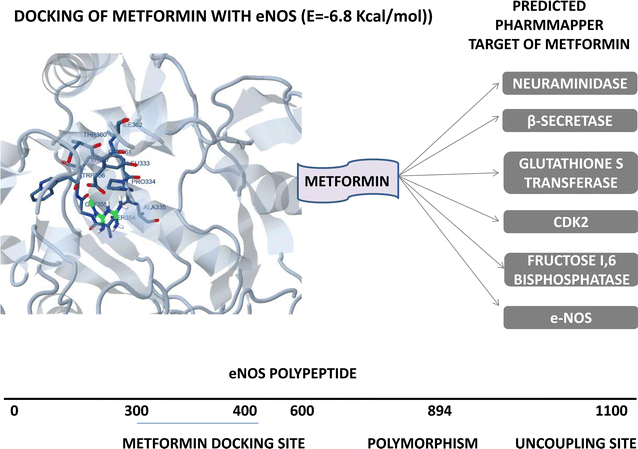
Conclusion: Finally, we conclude on eNOS-metformin-HSp90 signaling and its remedial effect for controlling the EPC to improve the diabetic condition for delaying diabetes-related complication. Altogether, the review gives a holistic overview about the elaborate therapeutic effect of EPC regulated by novel and existing drugs in diabetes and diabetes-related disorder.
Keywords: Diabetes; EPC; Metformin; NOX; ROS; eNOS.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

