IL-8 mediates idiopathic pulmonary fibrosis mesenchymal progenitor cell fibrogenicity
- PMID: 28860143
- PMCID: PMC5866425
- DOI: 10.1152/ajplung.00200.2017
IL-8 mediates idiopathic pulmonary fibrosis mesenchymal progenitor cell fibrogenicity
Abstract
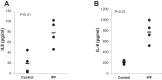
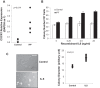
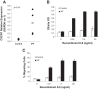
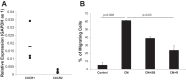
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic lung disease, but the mechanisms driving progression remain incompletely defined. We previously reported that the IPF lung harbors fibrogenic mesenchymal progenitor cells (MPCs), which serve as a cell of origin for IPF fibroblasts. Proliferating IPF MPCs are located at the periphery of fibroblastic foci in an active cellular front at the interface between the myofibroblast-rich focus core and adjacent normal alveolar structures. Among a large set of genes that distinguish IPF MPCs from their control counterparts, we identified IL-8 as a candidate mediator of IPF MPC fibrogenicity and driver of fibrotic progression. IPF MPCs and their progeny displayed increased steady-state levels of IL-8 and its cognate receptor CXCR1 and secreted more IL-8 than did controls. IL-8 functioned in an autocrine manner promoting IPF MPC self-renewal and the proliferation and motility of IPF MPC progeny. Secreted IL-8 also functioned in a paracrine manner stimulating macrophage migration. Analysis of IPF lung tissue demonstrated codistribution of IPF MPCs with activated macrophages in the active cellular front of the fibroblastic focus. These findings indicate that IPF MPC-derived IL-8 is capable of expanding the mesenchymal cell population and recruiting activated macrophages cells to actively evolving fibrotic lesions.
Keywords: IL-8; IPF mesenchymal progenitor cell; fibrotic front; macrophage.
Figures


Similar articles
-
IL-8 concurrently promotes idiopathic pulmonary fibrosis mesenchymal progenitor cell senescence and PD-L1 expression enabling escape from immune cell surveillance.Am J Physiol Lung Cell Mol Physiol. 2023 Jun 1;324(6):L849-L862. doi: 10.1152/ajplung.00028.2023. Epub 2023 Apr 25. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 37121574 Free PMC article.
-
Calcium-binding protein S100A4 confers mesenchymal progenitor cell fibrogenicity in idiopathic pulmonary fibrosis.J Clin Invest. 2017 Jun 30;127(7):2586-2597. doi: 10.1172/JCI90832. Epub 2017 May 22. J Clin Invest. 2017. PMID: 28530639 Free PMC article.
-
A CD44/Brg1 nuclear complex confers mesenchymal progenitor cells with enhanced fibrogenicity in idiopathic pulmonary fibrosis.JCI Insight. 2021 May 10;6(9):e144652. doi: 10.1172/jci.insight.144652. JCI Insight. 2021. PMID: 33822772 Free PMC article.
-
IL-25/IL-33/TSLP contributes to idiopathic pulmonary fibrosis: Do alveolar epithelial cells and (myo)fibroblasts matter?Exp Biol Med (Maywood). 2020 May;245(10):897-901. doi: 10.1177/1535370220915428. Epub 2020 Apr 4. Exp Biol Med (Maywood). 2020. PMID: 32249602 Free PMC article. Review.
-
Role of Mesenchymal Stem Cells and Extracellular Vesicles in Idiopathic Pulmonary Fibrosis.Int J Mol Sci. 2022 Sep 23;23(19):11212. doi: 10.3390/ijms231911212. Int J Mol Sci. 2022. PMID: 36232511 Free PMC article. Review.
Cited by
-
Promising Biomarkers of Radiation-Induced Lung Injury: A Review.Biomedicines. 2021 Sep 8;9(9):1181. doi: 10.3390/biomedicines9091181. Biomedicines. 2021. PMID: 34572367 Free PMC article. Review.
-
Development and Validation of the Prognostic Index Based on Inflammation-Related Gene Analysis in Idiopathic Pulmonary Fibrosis.Front Mol Biosci. 2021 Jul 22;8:667459. doi: 10.3389/fmolb.2021.667459. eCollection 2021. Front Mol Biosci. 2021. PMID: 34368225 Free PMC article.
-
Mesenchymal stem cells lineage and their role in disease development.Mol Med. 2024 Nov 11;30(1):207. doi: 10.1186/s10020-024-00967-9. Mol Med. 2024. PMID: 39523306 Free PMC article. Review.
-
Hyaluronan/CD44 axis regulates S100A4-mediated mesenchymal progenitor cell fibrogenicity in idiopathic pulmonary fibrosis.Am J Physiol Lung Cell Mol Physiol. 2021 May 1;320(5):L926-L941. doi: 10.1152/ajplung.00456.2020. Epub 2021 Mar 10. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33719561 Free PMC article.
-
Idiopathic pulmonary fibrosis (IPF): disease pathophysiology, targets, and potential therapeutic interventions.Mol Cell Biochem. 2024 Sep;479(9):2181-2194. doi: 10.1007/s11010-023-04845-6. Epub 2023 Sep 14. Mol Cell Biochem. 2024. PMID: 37707699 Review.
References
-
- American Thoracic Society American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 161: 646–664, 2000. doi:10.1164/ajrccm.161.2.ats3-00. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

