IPF lung fibroblasts have a senescent phenotype
- PMID: 28860144
- PMCID: PMC6148001
- DOI: 10.1152/ajplung.00220.2017
IPF lung fibroblasts have a senescent phenotype
Abstract
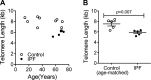
The mechanisms of aging that are involved in the development of idiopathic pulmonary fibrosis (IPF) are still unclear. Although it has been hypothesized that the proliferation and activation of human lung fibroblasts (hLFs) are essential in IPF, no studies have assessed how this process works in an aging lung. Our goal was to elucidate if there were age-related changes on primary hLFs isolated from IPF lungs compared with age-matched controls. We investigated several hallmarks of aging in hLFs from IPF patients and age-matched controls. IPF hLFs have increased cellular senescence with higher expression of β-galactosidase, p21, p16, p53, and cytokines related to the senescence-associated secretory phenotype (SASP) as well as decreased proliferation/apoptosis compared with age-matched controls. Additionally, we observed shorter telomeres, mitochondrial dysfunction, and upon transforming growth factor-β stimulation, increased markers of endoplasmic reticulum stress. Our data suggest that IPF hLFs develop senescence resulting in a decreased apoptosis and that the development of SASP may be an important contributor to the fibrotic process observed in IPF. These results might change the existing paradigm, which describes fibroblasts as aberrantly activated cells, to a cell with a senescence phenotype.
Keywords: TGF-β; aging; collagen; fibroblasts; idiopathic pulmonary fibrosis; mitochondria; senescence; telomeres.
Copyright © 2017 the American Physiological Society.
Figures





References
-
- Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA 3rd, Lansdorp PM, Loyd JE, Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 105: 13051–13056, 2008. doi: 10.1073/pnas.0804280105. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

