Improved Detection of Circulating Epithelial Cells in Patients with Intraductal Papillary Mucinous Neoplasms
- PMID: 28860411
- PMCID: PMC5759811
- DOI: 10.1634/theoncologist.2017-0234
Improved Detection of Circulating Epithelial Cells in Patients with Intraductal Papillary Mucinous Neoplasms
Erratum in
-
Improved Detection of Circulating Epithelial Cells in Patients with Intraductal Papillary Mucinous Neoplasms.Oncologist. 2018 Oct;23(10):1260. doi: 10.1634/theoncologist.2017-0234erratum. Oncologist. 2018. PMID: 30315083 Free PMC article. No abstract available.
Abstract
Background: Recent work has demonstrated early shedding of circulating epithelial cells (CECs) from premalignant intraductal papillary mucinous neoplasms (IPMNs). However, the potential use of CECs as a "liquid biopsy" for patients with IPMNs has been limited by antigen dependence of CEC isolation devices and the lack of robust detection biomarkers across CEC phenotypes.
Materials and methods: We utilized a negative depletion microfluidic platform to purify CECs from contaminating leukocytes and coupled this platform with immunofluorescence, RNA in situ hybridization, and RNA sequencing (RNA-seq) detection and enumeration.
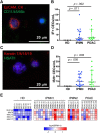
Results: Using established protein (EpCAM, cytokeratins) and novel noncoding RNA (HSATII, cytokeratins) biomarkers, we detected CECs in 88% of patients bearing IPMN lesions. RNA-seq analysis for MUC genes confirm the likely origin of these CECs from pancreatic lesions.
Conclusion: Our findings increase the sensitivity of detection of these cells and therefore could have clinical implications for cancer risk stratification.
Implications for practice: This work describes a high-sensitivity platform for detection of epithelial cells shed from preneoplastic lesions at high risk of malignant transformation. Further research efforts are underway to define the transcriptional programs that might allow discrimination between circulating cells released from tumors that will become malignant and cells released from tumors that will not. After further refinement, this combination of technologies could be deployed for monitoring and early detection of patients at high risk for developing new or recurrent pancreatic malignancies.
Keywords: Circulating epithelial cells; Early detection; Pancreatic cancer.
© AlphaMed Press 2017.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

Comment in
-
Regarding "Improved Detection of Circulating Epithelial Cells in Patients with Intraductal Papillary Mucinous Neoplasms".Oncologist. 2018 Oct;23(10):e119. doi: 10.1634/theoncologist.2018-0148. Epub 2018 Sep 4. Oncologist. 2018. PMID: 30181312 Free PMC article.
-
In Reply.Oncologist. 2018 Oct;23(10):e120. doi: 10.1634/theoncologist.2018-0374. Epub 2018 Oct 10. Oncologist. 2018. PMID: 30305416 Free PMC article.
References
-
- Rahib L, Smith BD, Aizenberg R et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–2921. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

