Mechanical basis of bone strength: influence of bone material, bone structure and muscle action
- PMID: 28860414
- PMCID: PMC5601257
Mechanical basis of bone strength: influence of bone material, bone structure and muscle action
Abstract
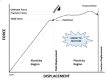
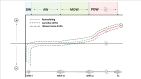
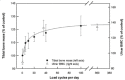
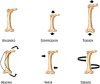
This review summarises current understanding of how bone is sculpted through adaptive processes, designed to meet the mechanical challenges it faces in everyday life and athletic pursuits, serving as an update for clinicians, researchers and physical therapists. Bone's ability to resist fracture under the large muscle and locomotory forces it experiences during movement and in falls or collisions is dependent on its established mechanical properties, determined by bone's complex and multidimensional material and structural organisation. At all levels, bone is highly adaptive to habitual loading, regulating its structure according to components of its loading regime and mechanical environment, inclusive of strain magnitude, rate, frequency, distribution and deformation mode. Indeed, the greatest forces habitually applied to bone arise from muscular contractions, and the past two decades have seen substantial advances in our understanding of how these forces shape bone throughout life. Herein, we also highlight the limitations of in vivo methods to assess and understand bone collagen, and bone mineral at the material or tissue level. The inability to easily measure or closely regulate applied strain in humans is identified, limiting the translation of animal studies to human populations, and our exploration of how components of mechanical loading regimes influence mechanoadaptation.
Conflict of interest statement
The authors have no conflict of interest.
Figures




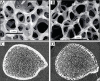

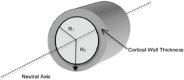
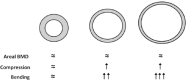
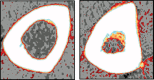
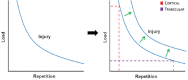
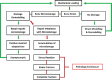
References
-
- Ray R, Clement ND, Aitken SA, McQueen MM, Court-Brown CM, Ralston SH. High mortality in younger patients with major osteoporotic fractures. Osteoporos Int. 2017;28(3):1047–52. - PubMed
-
- Milte R, Crotty M. Musculoskeletal health, frailty and functional decline. Best Pract Res Clin Rheumatol. 2014;28(3):395–410. - PubMed
-
- Burr DB, Forwood MR, Fyhrie DP, Martin RB, Schaffler MB, Turner CH. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res. 1997;12(1):6–15. - PubMed
-
- Hart NH, Nimphius S, Weber J, Dobbin M, Newton RU. Lower body bone mass characteristics of elite, sub-elite and amateur Australian Footballers. J Aust Str Cond. 2013;21(1):50–3.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
