Bacterial flagellin-a potent immunomodulatory agent
- PMID: 28860663
- PMCID: PMC5628280
- DOI: 10.1038/emm.2017.172
Bacterial flagellin-a potent immunomodulatory agent
Abstract
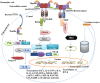
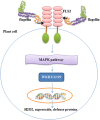
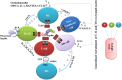
Flagellin is a subunit protein of the flagellum, a whip-like appendage that enables bacterial motility. Traditionally, flagellin was viewed as a virulence factor that contributes to the adhesion and invasion of host cells, but now it has emerged as a potent immune activator, shaping both the innate and adaptive arms of immunity during microbial infections. In this review, we summarize our understanding of bacterial flagellin and host immune system interactions and the role flagellin as an adjuvant, anti-tumor and radioprotective agent, and we address important areas of future research interests.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol 2001; 2: 947–950. - PubMed
-
- Liew FY, Xu D, Brint EK, O’Neill LAJ. Negative regulation of Toll-like receptor-mediated immune responses. Nat Rev Immunol 2005; 5: 446–458. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006; 124: 783–801. - PubMed
-
- Medzhitov R, Janeway C. Innate immune recognition: mechanisms and pathways. Immunol Rev 2000; 173: 89–97. - PubMed
-
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011; 34: 637–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

