A randomized, double-blind study of hydromorphone hydrochloride extended-release tablets versus oxycodone hydrochloride extended-release tablets for cancer pain: efficacy and safety in Japanese cancer patients (EXHEAL: a Phase III study of EXtended-release HydromorphonE for cAncer pain reLief)
- PMID: 28860850
- PMCID: PMC5571837
- DOI: 10.2147/JPR.S136937
A randomized, double-blind study of hydromorphone hydrochloride extended-release tablets versus oxycodone hydrochloride extended-release tablets for cancer pain: efficacy and safety in Japanese cancer patients (EXHEAL: a Phase III study of EXtended-release HydromorphonE for cAncer pain reLief)
Abstract
Background: In Japan, there are limited options for switching opioid analgesics. Hydromorphone is an opioid analgesic that is routinely used instead of morphine for cancer pain; however, it is not yet available in Japan. The aim of this study was to assess the efficacy and safety of hydromorphone (DS-7113b) extended-release tablets in opioid-naïve patients with cancer pain not relieved by non-opioid analgesics.
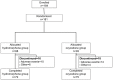
Subjects and methods: This was a multicenter, randomized, double-blind, parallel-group trial. A double-dummy method was used for blinding. Each randomized subject received either hydromorphone extended-release tablets plus placebo oxycodone hydrochloride extended-release tablets 4 mg/day (n=88) or placebo hydromorphone extended-release tablets plus oxycodone hydrochloride extended-release tablets 10 mg/day (n=93) orally for 7 days (once-daily dosing for hydromorphone and twice-daily dosing for oxycodone). The doses were adjusted as necessary. Efficacy was evaluated by change in visual analog scale (VAS) score from baseline to completion of treatment.
Results: The between-group difference in least squares mean changes in VAS score from baseline to completion or discontinuation of treatment was -0.4 mm (95% CI -5.9 to 5 mm) by analysis of covariance where the baseline VAS score was used as a covariate. The upper limit of the 95% CI was below 10 mm, which was predefined as the noninferiority limit. This verified the noninferiority of hydromorphone tablets relative to oxycodone tablets. The incidence of adverse events was 80.7% (71 of 88) in the hydromorphone group and 83.7% (77 of 93) in the oxycodone group. The most common adverse events were nausea, vomiting, somnolence, diarrhea, and constipation, most of which are commonly observed with opioid analgesics.
Conclusion: The efficacy and safety of hydromorphone extended-release tablets were equivalent to those of the oxycodone extended-release formulation.
Keywords: cancer pain; double-blind study; hydromorphone; oxycodone; palliative medicine.
Conflict of interest statement
Disclosure SI, AI, and YK are employees of Daiichi Sankyo. YS participated in this study as a medical specialist, and ST and EA functioned as safety assessment advisors. YS has received personal fees from Daiichi Sankyo. The authors report no other conflicts of interest in this work.
Figures

References
-
- World Health Organization . Cancer Pain Relief: With a Guide to Opioid Availability. 2nd ed. Geneva: WHO; 1996.
-
- Zech DF, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of World Health Organization guidelines for cancer pain relief: a 10-year prospective study. Pain. 1995;63(1):65–76. - PubMed
-
- International Narcotics Control Board . Report of the International Narcotics Control Board for 2013. Vienna: United Nations; 2014.
-
- Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–e68. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

