Long-Lasting Enhancement of Visual Perception with Repetitive Noninvasive Transcranial Direct Current Stimulation
- PMID: 28860969
- PMCID: PMC5559806
- DOI: 10.3389/fncel.2017.00238
Long-Lasting Enhancement of Visual Perception with Repetitive Noninvasive Transcranial Direct Current Stimulation
Abstract
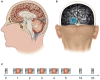
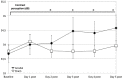
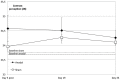
Understanding processes performed by an intact visual cortex as the basis for developing methods that enhance or restore visual perception is of great interest to both researchers and medical practitioners. Here, we explore whether contrast sensitivity, a main function of the primary visual cortex (V1), can be improved in healthy subjects by repetitive, noninvasive anodal transcranial direct current stimulation (tDCS). Contrast perception was measured via threshold perimetry directly before and after intervention (tDCS or sham stimulation) on each day over 5 consecutive days (24 subjects, double-blind study). tDCS improved contrast sensitivity from the second day onwards, with significant effects lasting 24 h. After the last stimulation on day 5, the anodal group showed a significantly greater improvement in contrast perception than the sham group (23 vs. 5%). We found significant long-term effects in only the central 2-4° of the visual field 4 weeks after the last stimulation. We suspect a combination of two factors contributes to these lasting effects. First, the V1 area that represents the central retina was located closer to the polarization electrode, resulting in higher current density. Second, the central visual field is represented by a larger cortical area relative to the peripheral visual field (cortical magnification). This is the first study showing that tDCS over V1 enhances contrast perception in healthy subjects for several weeks. This study contributes to the investigation of the causal relationship between the external modulation of neuronal membrane potential and behavior (in our case, visual perception). Because the vast majority of human studies only show temporary effects after single tDCS sessions targeting the visual system, our study underpins the potential for lasting effects of repetitive tDCS-induced modulation of neuronal excitability.
Keywords: contrast sensitivity; noninvasive brain stimulation; plasticity; primary visual cortex; transcranial direct current stimulation; visual perceptual learning.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

