Nanobubbles Form at Active Hydrophobic Spots on the Luminal Aspect of Blood Vessels: Consequences for Decompression Illness in Diving and Possible Implications for Autoimmune Disease-An Overview
- PMID: 28861003
- PMCID: PMC5559548
- DOI: 10.3389/fphys.2017.00591
Nanobubbles Form at Active Hydrophobic Spots on the Luminal Aspect of Blood Vessels: Consequences for Decompression Illness in Diving and Possible Implications for Autoimmune Disease-An Overview
Abstract
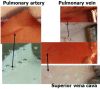
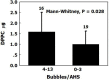
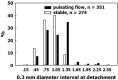
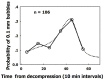
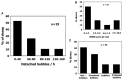
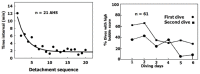
Decompression illness (DCI) occurs following a reduction in ambient pressure. Decompression bubbles can expand and develop only from pre-existing gas micronuclei. The different hypotheses hitherto proposed regarding the nucleation and stabilization of gas micronuclei have never been validated. It is known that nanobubbles form spontaneously when a smooth hydrophobic surface is submerged in water containing dissolved gas. These nanobubbles may be the long sought-after gas micronuclei underlying decompression bubbles and DCI. We exposed hydrophobic and hydrophilic silicon wafers under water to hyperbaric pressure. After decompression, bubbles appeared on the hydrophobic but not the hydrophilic wafers. In a further series of experiments, we placed large ovine blood vessels in a cooled high pressure chamber at 1,000 kPa for about 20 h. Bubbles evolved at definite spots in all the types of blood vessels. These bubble-producing spots stained positive for lipids, and were henceforth termed "active hydrophobic spots" (AHS). The lung surfactant dipalmitoylphosphatidylcholine (DPPC), was found both in the plasma of the sheep and at the AHS. Bubbles detached from the blood vessel in pulsatile flow after reaching a mean diameter of ~1.0 mm. Bubble expansion was bi-phasic-a slow initiation phase which peaked 45 min after decompression, followed by fast diffusion-controlled growth. Many features of decompression from diving correlate with this finding of AHS on the blood vessels. (1) Variability between bubblers and non-bubblers. (2) An age-related effect and adaptation. (3) The increased risk of DCI on a second dive. (4) Symptoms of neurologic decompression sickness. (5) Preconditioning before a dive. (6) A bi-phasic mechanism of bubble expansion. (7) Increased bubble formation with depth. (8) Endothelial injury. (9) The presence of endothelial microparticles. Finally, constant contact between nanobubbles and plasma may result in distortion of proteins and their transformation into autoantigens.
Keywords: decompression illness; endothel; nucleation; sheep; stabilization; surfactant.
Figures







Comment in
-
Letter to the Editor re: Evolution and Preservation of Venous Gas Emboli at Alternating High and Moderate Altitude Exposures: Letters.Aerosp Med Hum Perform. 2020 May 1;91(5):455. doi: 10.3357/AMHP.5579.2020. Aerosp Med Hum Perform. 2020. PMID: 32327021 No abstract available.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

