Characterization of Histopathological and Ultrastructural Changes in Channel Catfish Experimentally Infected with Virulent Aeromonas hydrophila
- PMID: 28861049
- PMCID: PMC5559642
- DOI: 10.3389/fmicb.2017.01519
Characterization of Histopathological and Ultrastructural Changes in Channel Catfish Experimentally Infected with Virulent Aeromonas hydrophila
Abstract
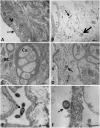
A highly virulent clonal population of Aeromonas hydrophila (vAh) has been the cause of recent motile Aeromonas septicemia epizootic in channel catfish (Ictalurus punctatus) farms in the Southeastern United States. The pathology of the disease caused by vAh has not been studied well yet. Thus, our aim was to determine histopathological and ultrastructural changes in channel catfish following vAh challenge. To accomplish this, catfish fingerlings were challenged with vAh (strain ML09-119) by bath. Six fish per each time point were collected at 1, 3, 5, 6, 24, and 48 h for light microscopy, and six fish were collected at 48 h for transmission electron microscopy (TEM). The first pathological lesions were detected in the spleen and stomach at 1 h post-challenge (HPC) while intestine, gills, kidney, and liver lesions were observed at 24 and 48 HPC. Histopathological examination revealed degenerative changes, necrosis, extensive edema, and inflammation in internal organs. The TEM showed severe tissue destruction with multiple bacterial cells secreting outer membrane vesicles, especially in spleen and gills and far number in the stomach. Degenerated bacterial cells were observed in the intestinal lumen and the phagosomes of phagocytic kidney cells. We identified, for the first time, degranulate eosinophilic granular cells, and dendritic cells like (DC-like) cells in the necrotic intestinal epithelium. These findings suggest that vAh rapidly proliferated and spread through the catfish organs following bath challenge.
Keywords: DC-like cells; electron microscopy; eosinophilic granular cells; histopathology; vAh.
Figures











References
-
- Afifi S. H., Al-Thobiati S., Hazaa M. S. (2000). Bacteriological and histopathological studies on Aeromonas hydrophila infection of Nile tilapia (Oreochromis niloticus) from fish farms in Saudi Arabia. Assiut Vet. Med. J. 42 195–205.
-
- Alagappan K. M., Deivasigamani B., Kumaran S., Sakthivel M. (2009). Histopathological alterations in estuarine catfish (Arius maculatus; Thunberg, 1792) due to Aeronomas hydrophila infection. World J. Fish Mar. Sci. 1 185–189.
-
- Andrea G. M., Edgar Oliver Lopez V., Aurora Longa B., Castro-Escarpulli G. (2015). Production of outer membrane vesicles in a clinical strain of Aeromonas hydrophila. Microbiology 1 113–117.
-
- Angka S. (1990). The pathology of the walking catfish, Clarias batrachus (L.), infected intraperitoneally with Aeromonas hydrophila. Asian Fish. Sci. 3 343–351.
LinkOut - more resources
Full Text Sources
Other Literature Sources

