Tacrolimus in the treatment of myasthenia gravis in patients with an inadequate response to glucocorticoid therapy: randomized, double-blind, placebo-controlled study conducted in China
- PMID: 28861121
- PMCID: PMC5557184
- DOI: 10.1177/1756285617721092
Tacrolimus in the treatment of myasthenia gravis in patients with an inadequate response to glucocorticoid therapy: randomized, double-blind, placebo-controlled study conducted in China
Abstract
Background: To determine the efficacy of low-dose, immediate-release tacrolimus in patients with myasthenia gravis (MG) with inadequate response to glucocorticoid therapy in a randomized, double-blind, placebo-controlled study.
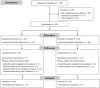
Methods: Eligible patients had inadequate response to glucocorticoids (GCs) after ⩾6 weeks of treatment with prednisone ⩾0.75 mg/kg/day or 60-100 mg/day. Patients were randomized to receive 3 mg tacrolimus or placebo daily (orally) for 24 weeks. Concomitant glucocorticoids and pyridostigmine were allowed. Patients continued GC therapy from weeks 1-4; from week 5, the dose was decreased at the discretion of the investigator. The primary efficacy outcome measure was a reduction, relative to baseline, in quantitative myasthenia gravis (QMG) score assessed using a generalized linear model; supportive analyses used alternative models.
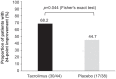
Results: Of 138 patients screened, 83 [tacrolimus (n = 45); placebo (n = 38)] were enrolled and treated. The change in adjusted mean QMG score from baseline to week 24 was -4.9 for tacrolimus and -3.3 for placebo (least squares mean difference: -1.7, 95% confidence interval: -3.5, -0.1; p = 0.067). A post-hoc analysis demonstrated a statistically significant difference for QMG score reduction of ⩾4 points in the tacrolimus group (68.2%) versus the placebo group (44.7%; p = 0.044). Adverse event profiles were similar between treatment groups.
Conclusions: Tacrolimus 3 mg treatment for patients with MG and inadequate response to GCs did not demonstrate a statistically significant improvement in the primary endpoint versus placebo over 24 weeks; however, a post-hoc analysis demonstrated a statistically significant difference for QMG score reduction of ⩾4 points in the tacrolimus group versus the placebo group. This study was limited by the low number of patients, the absence of testing for acetylcholine receptor antibody and the absence of stratification by disease duration (which led to a disparity between the two groups). ClinicalTrials.gov identifier: NCT01325571.
Keywords: immunology; myasthenia gravis; tacrolimus.
Conflict of interest statement
Conflict of interest statement: Huawei Shi is an employee of Astellas Pharma China, Inc. All other authors declare no conflict of interest.
Figures

Similar articles
-
Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study.Lancet Neurol. 2017 Dec;16(12):976-986. doi: 10.1016/S1474-4422(17)30369-1. Epub 2017 Oct 20. Lancet Neurol. 2017. PMID: 29066163 Clinical Trial.
-
Clinical Effects of the Self-administered Subcutaneous Complement Inhibitor Zilucoplan in Patients With Moderate to Severe Generalized Myasthenia Gravis: Results of a Phase 2 Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Trial.JAMA Neurol. 2020 May 1;77(5):582-592. doi: 10.1001/jamaneurol.2019.5125. JAMA Neurol. 2020. PMID: 32065623 Free PMC article. Clinical Trial.
-
Randomized study of adjunctive belimumab in participants with generalized myasthenia gravis.Neurology. 2018 Apr 17;90(16):e1425-e1434. doi: 10.1212/WNL.0000000000005323. Epub 2018 Mar 21. Neurology. 2018. PMID: 29661905 Free PMC article. Clinical Trial.
-
Effectiveness and safety of tacrolimus therapy for myasthenia gravis: A single arm meta-analysis.J Clin Neurosci. 2019 May;63:160-167. doi: 10.1016/j.jocn.2019.02.004. Epub 2019 Mar 1. J Clin Neurosci. 2019. PMID: 30827886
-
Efficacy and safety of low-dose rituximab in the treatment of myasthenia gravis: a systemic review and meta-analysis.Front Neurol. 2024 Sep 25;15:1439899. doi: 10.3389/fneur.2024.1439899. eCollection 2024. Front Neurol. 2024. PMID: 39385818 Free PMC article.
Cited by
-
Long-Term Improvement in a Chinese Cohort of Glucocorticoid-Resistant Childhood-Onset Myasthenia Gravis Patients Treated With Tacrolimus.Front Neurol. 2022 Feb 8;13:820205. doi: 10.3389/fneur.2022.820205. eCollection 2022. Front Neurol. 2022. PMID: 35211085 Free PMC article.
-
Maintenance of zilucoplan efficacy in patients with generalised myasthenia gravis up to 24 weeks: a model-informed analysis.Ther Adv Neurol Disord. 2024 Sep 21;17:17562864241279125. doi: 10.1177/17562864241279125. eCollection 2024. Ther Adv Neurol Disord. 2024. PMID: 39314260 Free PMC article.
-
Efficacy and Safety of Tacrolimus Therapy for a Single Chinese Cohort With Very-Late-Onset Myasthenia Gravis.Front Neurol. 2022 Mar 30;13:843523. doi: 10.3389/fneur.2022.843523. eCollection 2022. Front Neurol. 2022. PMID: 35432159 Free PMC article.
-
Efficacy and safety of tacrolimus for myasthenia gravis: a systematic review and meta-analysis.J Neurol. 2017 Nov;264(11):2191-2200. doi: 10.1007/s00415-017-8616-7. Epub 2017 Sep 18. J Neurol. 2017. PMID: 28921038
-
Effects of Mitophagy on Regulatory T Cell Function in Patients With Myasthenia Gravis.Front Neurol. 2020 Apr 7;11:238. doi: 10.3389/fneur.2020.00238. eCollection 2020. Front Neurol. 2020. PMID: 32318017 Free PMC article.
References
-
- Gilhus NE. Myasthenia gravis. New Engl J Med 2016; 375: 2570–2581. - PubMed
-
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015; 14: 1023–1036. - PubMed
-
- Jaretzki A, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 2000; 55: 16–23. - PubMed
-
- Guo J, Dang D, Li H-Z, et al. Current overview of myasthenia gravis and experience in China. Neuroimmunol Neuroinflammation 2014; 1: 127–130.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

