Comparison of periodontitis-associated oral biofilm formation under dynamic and static conditions
- PMID: 28861286
- PMCID: PMC5577440
- DOI: 10.5051/jpis.2017.47.4.219
Comparison of periodontitis-associated oral biofilm formation under dynamic and static conditions
Abstract
Purpose: The purpose of this study was to compare the characteristics of single- and dual-species in vitro oral biofilms made by static and dynamic methods.
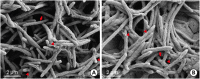
Methods: Hydroxyapatite (HA) disks, 12.7 mm in diameter and 3 mm thick, were coated with processed saliva for 4 hours. The disks were divided into a static method group and a dynamic method group. The disks treated with a static method were cultured in 12-well plates, and the disks in the dynamic method group were cultured in a Center for Disease Control and Prevention (CDC) biofilm reactor for 72 hours. In the single- and dual-species biofilms, Fusobacterium nucleatum and Porphyromonas gingivalis were used, and the amount of adhering bacteria, proportions of species, and bacterial reduction of chlorhexidine were examined. Bacterial adhesion was examined with scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM).
Results: Compared with the biofilms made using the static method, the biofilms made using the dynamic method had significantly lower amounts of adhering and looser bacterial accumulation in SEM and CLSM images. The proportion of P. gingivalis was higher in the dynamic method group than in the static method group; however, the difference was not statistically significant. Furthermore, the biofilm thickness and bacterial reduction by chlorhexidine showed no significant differences between the 2 methods.
Conclusions: When used to reproduce periodontal biofilms composed of F. nucleatum and P. gingivalis, the dynamic method (CDC biofilm reactor) formed looser biofilms containing fewer bacteria than the well plate. However, this difference did not influence the thickness of the biofilms or the activity of chlorhexidine. Therefore, both methods are useful for mimicking periodontitis-associated oral biofilms.
Keywords: Bacterial adhesion; Biofilms; Chlorhexidine; Electron microscope tomography; Periodontitis.
Conflict of interest statement
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Figures



References
-
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. - PubMed
-
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. - PubMed
-
- Filoche S, Wong L, Sissons CH. Oral biofilms: emerging concepts in microbial ecology. J Dent Res. 2010;89:8–18. - PubMed
-
- Ximénez-Fyvie LA, Haffajee AD, Socransky SS. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol. 2000;27:648–657. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

