Clinical neuroimaging in the preterm infant: Diagnosis and prognosis
- PMID: 28861337
- PMCID: PMC5568883
- DOI: 10.1016/j.nicl.2017.08.015
Clinical neuroimaging in the preterm infant: Diagnosis and prognosis
Abstract
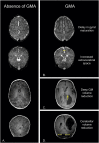
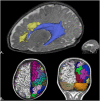
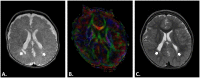
Perinatal care advances emerging over the past twenty years have helped to diminish the mortality and severe neurological morbidity of extremely and very preterm neonates (e.g., cystic Periventricular Leukomalacia [c-PVL] and Germinal Matrix Hemorrhage - Intraventricular Hemorrhage [GMH-IVH grade 3-4/4]; 22 to < 32 weeks of gestational age, GA). However, motor and/or cognitive disabilities associated with mild-to-moderate white and gray matter injury are frequently present in this population (e.g., non-cystic Periventricular Leukomalacia [non-cystic PVL], neuronal-axonal injury and GMH-IVH grade 1-2/4). Brain research studies using magnetic resonance imaging (MRI) report that 50% to 80% of extremely and very preterm neonates have diffuse white matter abnormalities (WMA) which correspond to only the minimum grade of severity. Nevertheless, mild-to-moderate diffuse WMA has also been associated with significant affectations of motor and cognitive activities. Due to increased neonatal survival and the intrinsic characteristics of diffuse WMA, there is a growing need to study the brain of the premature infant using non-invasive neuroimaging techniques sensitive to microscopic and/or diffuse lesions. This emerging need has led the scientific community to try to bridge the gap between concepts or ideas from different methodologies and approaches; for instance, neuropathology, neuroimaging and clinical findings. This is evident from the combination of intense pre-clinical and clinicopathologic research along with neonatal neurology and quantitative neuroimaging research. In the following review, we explore literature relating the most frequently observed neuropathological patterns with the recent neuroimaging findings in preterm newborns and infants with perinatal brain injury. Specifically, we focus our discussions on the use of neuroimaging to aid diagnosis, measure morphometric brain damage, and track long-term neurodevelopmental outcomes.
Keywords: Preterm neonates; infants; magnetic resonance imaging; neonatal neurology; perinatal brain injury; white matter abnormalities.
Figures


References
-
- Ajayi-Obe M., Saeed N., Cowan F., Rutherford M., Edwards A. Reduced development of cerebral cortex in extremely preterm infants. Lancet. 2000;356(9236):1162–1163. - PubMed
-
- Ancel P.-Y., Goffinet F., Kuhn P., Langer B., Matis J., Hernandorena X.…Kaminski M. Survival and morbidity of preterm children born at 22 through 34 weeks' gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. 2015;169(3):230–238. - PubMed
-
- Anderson P.J., Cheong J.L.Y., Thompson D.K. The predictive validity of neonatal MRI for neurodevelopmental outcome in very preterm children. Semin. Perinatol. 2015;39(2):147–158. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

