Rapid identification of health care-associated infections with an integrated fluorescence anisotropy system
- PMID: 28861468
- PMCID: PMC5565941
- DOI: 10.1126/sciadv.1600300
Rapid identification of health care-associated infections with an integrated fluorescence anisotropy system
Abstract
Health care-associated infections (HAIs) and drug-resistant pathogens have become a major health care issue with millions of reported cases every year. Advanced diagnostics would allow clinicians to more quickly determine the most effective treatment, reduce the nonspecific use of broad-spectrum antimicrobials, and facilitate enrollment in new antibiotic treatments. We present a new integrated system, polarization anisotropy diagnostics (PAD), for rapid detection of HAI pathogens. The PAD uses changes of fluorescence anisotropy when detection probes recognize target bacterial nucleic acids. The technology is inherently robust against environmental noise and economically scalable for parallel measurements. The assay is fast (2 hours) and performed on-site in a single-tube format. When applied to clinical samples obtained from interventional procedures, the PAD determined the overall bacterial burden, differentiated HAI bacterial species, and identified drug resistance and virulence status. The PAD system holds promise as a powerful tool for near-patient, rapid HAI testing.
Keywords: Biosensors; antibiotics; antimicrobials; drug resistance; fluorescence anisotropy; healthcare–associated infections; nanotechnology; nucleic acid testing; superbugs.
Figures


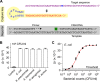



References
-
- Magill S. S., Edwards J. R., Bamberg W., Beldavs Z. G., Dumyati G., Kainer M. A., Lynfield R., Maloney M., McAllister-Hollod L., Nadle J., Ray S. M., Thompson D. L., Wilson L. E., Fridkin S. K.; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team , Multistate point-prevalence survey of health care–associated infections. N. Engl. J. Med. 370, 1198–1208 (2014). - PMC - PubMed
-
- Marchetti A., Rossiter R., Economic burden of healthcare-associated infection in US acute care hospitals: Societal perspective. J. Med. Econ. 16, 1399–1404 (2013). - PubMed
-
- Allegranzi B., Bagheri Nejad S., Combescure C., Graafmans W., Attar H., Donaldson L., Pittet D., Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 377, 228–241 (2011). - PubMed
-
- Polin R. A., Denson S., Brady M. T.; Committee on Fetus and Newborn; Committee on Infectious Diseases , Epidemiology and diagnosis of health care–associated infections in the NICU. Pediatrics 129, e1104–e1109 (2012). - PubMed
-
- Klompas M., Yokoe D. S., Weinstein R. A., Automated surveillance of health care–associated infections. Clin. Infect. Dis. 48, 1268–1275 (2009). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

