Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy
- PMID: 28861484
- PMCID: PMC5576600
- DOI: 10.1089/can.2015.0012
Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy
Abstract
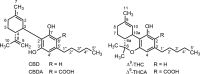
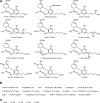
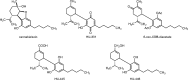
Cannabidiol (CBD), the main nonpsychoactive constituent of Cannabis sativa, has shown a wide range of therapeutically promising pharmacological effects either as a sole drug or in combination with other drugs in adjunctive therapy. However, the targets involved in the therapeutic effects of CBD appear to be elusive. Furthermore, scarce information is available on the biological activity of its human metabolites which, when formed in pharmacologically relevant concentration, might contribute to or even account for the observed therapeutic effects. The present overview summarizes our current knowledge on the pharmacokinetics and metabolic fate of CBD in humans, reviews studies on the biological activity of CBD metabolites either in vitro or in vivo, and discusses relevant drug-drug interactions. To facilitate further research in the area, the reported syntheses of CBD metabolites are also catalogued.
Keywords: biological activity; cannabidiol; metabolites; pharmacokinetics; synthesis.
Conflict of interest statement
No competing financial interest.
Figures
References
-
- Adams R, Hunt M, Clark JH. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. I. J Am Chem Soc. 1940;62:196–200
-
- Jacob A, Todd AR. Cannabis indica. Part II. Isolation of cannabidiol from Egyptian hashish. Observations on the structure of cannabinol. J Chem Soc. 1940;649–653
-
- Mechoulam R, Shvo Y. Hashish–I. The structure of cannabidiol. Tetrahedron. 1963;19:2073–2078 - PubMed
-
- ElSohly M, Gul W. Constituents of Cannabis sativa. In: Handbook of Cannabis (Pertwee RG, ed.). Oxford University Press: Oxford, 2014, pp. 3–22
-
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

