Butyrate Supplementation at High Concentrations Alters Enteric Bacterial Communities and Reduces Intestinal Inflammation in Mice Infected with Citrobacter rodentium
- PMID: 28861518
- PMCID: PMC5566833
- DOI: 10.1128/mSphere.00243-17
Butyrate Supplementation at High Concentrations Alters Enteric Bacterial Communities and Reduces Intestinal Inflammation in Mice Infected with Citrobacter rodentium
Abstract
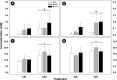
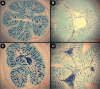
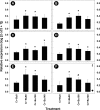
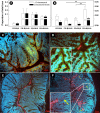
Butyrate is a short-chain fatty acid by-product of the microbial fermentation of dietary fermentable materials in the large intestine; it is the main energy source for enterocyte regeneration, modulates the enteric microbial community, and contributes to increasing host health via mechanisms that are relatively poorly defined. Limited research has examined the therapeutic potential of butyrate using models of enteric inflammation incited by pathogenic organisms. We used Citrobacter rodentium to incite acute Th1/Th17 inflammation to ascertain the impact of butyrate on the host-microbiota relationship. Rectal administration of 140 mM butyrate to mice increased fecal concentrations of butyrate and increased food consumption and weight gain in mice infected with C. rodentium. Histological scores of colonic inflammation were lower in infected mice administered 140 mM butyrate. Expression of Il10, Tgfβ, and Muc2 was elevated in noninfected mice administered butyrate in comparison to mice not administered butyrate. Infected mice administered butyrate displayed elevated expression of genes necessary for pathogen clearance (i.e., Il17A and Il1β) and of genes involved in epithelial barrier repair and restoration (i.e., Relmβ, Tff3, and Myd88). Butyrate supplemented to inflamed colons increased the abundances of Proteobacteria and Lachnospiraceae and reduced the abundance of Clostridiaceae species. Mice with enteritis that were administered butyrate also exhibited an increased abundance of mucus-associated bacteria. In summary, rectal administration of butyrate increased feed consumption and weight gain, ameliorated C. rodentium-induced cell injury through enhanced expression of immune regulation and tissue repair mechanisms, and increased the abundance of butyrate-producing bacteria in mice with enteritis. IMPORTANCE The study findings provide evidence that administration of butyrate in a dose-dependent manner can increase weight gain in infected mice, enhance clearance of the infection, reduce inflammation through altered cytokine expression, and enhance tissue repair and mucus secretion. Moreover, butyrate treatment also affected the abundance of bacterial populations in both noninflamed and inflamed intestines. Notably, this investigation provides foundational information that can be used to determine the effects of prebiotics and other functional foods on the production of butyrate by enteric bacteria and their impact on intestinal health and host well-being.
Keywords: Citrobacter rodentium; butyrate; inflammation; intestine; mice; microbiota.
Figures









References
-
- Lin HV, Frassetto A, Kowalik EJ Jr, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ. 2012. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 7:e35240. doi:10.1371/journal.pone.0035240. - DOI - PMC - PubMed
-
- Cummings JH. 1984. Colonic absorption: the importance of short chain fatty acids in man. Scand J Gastroenterol Suppl 93:89–99. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
