The immune response to secondary necrotic cells
- PMID: 28861714
- PMCID: PMC5630647
- DOI: 10.1007/s10495-017-1413-z
The immune response to secondary necrotic cells
Abstract
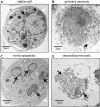
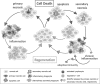
When apoptotic cells are not cleared in an efficient and timely manner, they progress to secondary necrosis and lose their membrane integrity. This results in a leakage of immunostimulatory, danger associated molecular patterns (DAMPs), similar to accidental (or primary) necrosis. However, primary necrosis is a sudden event with an inadvertent release of almost unmodified DAMPs. Secondary necrotic cells, in contrast, have gone through various modifications during the process of apoptosis. Recent research revealed that the molecules released from the cytoplasm or exposed on the cell surface differ between primary necrosis, secondary necrosis, and regulated necrosis such as necroptosis. This review gives an overview of these differences and focusses their effects on the immune response. The implications to human physiology and diseases are manifold and will be discussed in the context of cancer, neurodegenerative disorders and autoimmunity.
Keywords: Apoptosis; Cancer immunotherapy; Efferocytosis; Inflammation; Primary necrosis; Secondary necrosis.
Figures

Similar articles
-
Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system.FEBS J. 2016 Jul;283(14):2599-615. doi: 10.1111/febs.13775. Epub 2016 Jun 29. FEBS J. 2016. PMID: 27273805
-
How Kidney Cell Death Induces Renal Necroinflammation.Semin Nephrol. 2016 May;36(3):162-73. doi: 10.1016/j.semnephrol.2016.03.004. Semin Nephrol. 2016. PMID: 27339382 Review.
-
Inflammatory outcomes of apoptosis, necrosis and necroptosis.Biol Chem. 2014 Oct;395(10):1163-71. doi: 10.1515/hsz-2014-0164. Biol Chem. 2014. PMID: 25153241 Review.
-
Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses.Immunol Rev. 2017 Nov;280(1):126-148. doi: 10.1111/imr.12574. Immunol Rev. 2017. PMID: 29027218 Review.
-
Origin and Consequences of Necroinflammation.Physiol Rev. 2018 Apr 1;98(2):727-780. doi: 10.1152/physrev.00041.2016. Physiol Rev. 2018. PMID: 29465288 Review.
Cited by
-
Nrf2 regulates the arginase 1+ microglia phenotype through the initiation of TREM2 transcription, ameliorating depression-like behavior in mice.Transl Psychiatry. 2022 Oct 31;12(1):459. doi: 10.1038/s41398-022-02227-y. Transl Psychiatry. 2022. PMID: 36316319 Free PMC article.
-
Antiproliferative and Cytotoxic Properties of Propynoyl Betulin Derivatives against Human Ovarian Cancer Cells: In Vitro Studies.Int J Mol Sci. 2023 Nov 18;24(22):16487. doi: 10.3390/ijms242216487. Int J Mol Sci. 2023. PMID: 38003677 Free PMC article.
-
Lytic Cell Death Mechanisms in Human Respiratory Syncytial Virus-Infected Macrophages: Roles of Pyroptosis and Necroptosis.Viruses. 2020 Aug 25;12(9):932. doi: 10.3390/v12090932. Viruses. 2020. PMID: 32854254 Free PMC article.
-
Simulated in vitro hypoxic conditions from psoriatic arthritis cartilage change plasminogen activating system urokinase and serpine functionality. Reversal of antiapoptotic protection suggests common homeostatic buffering.Postepy Dermatol Alergol. 2022 Oct;39(5):944-952. doi: 10.5114/ada.2022.113405. Epub 2022 Feb 8. Postepy Dermatol Alergol. 2022. PMID: 36457693 Free PMC article.
-
Efferocytosis and Its Role in Inflammatory Disorders.Front Cell Dev Biol. 2022 Feb 25;10:839248. doi: 10.3389/fcell.2022.839248. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35281078 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

