Differential Effects of Antiretroviral Drugs on Neurons In Vitro: Roles for Oxidative Stress and Integrated Stress Response
- PMID: 28861811
- PMCID: PMC5821494
- DOI: 10.1007/s11481-017-9761-6
Differential Effects of Antiretroviral Drugs on Neurons In Vitro: Roles for Oxidative Stress and Integrated Stress Response
Abstract
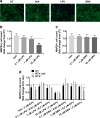
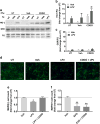
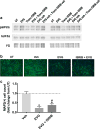
Mounting evidence suggests that antiretroviral drugs may contribute to the persistence of HIV-associated neurocognitive disorders (HAND), which impact 30%-50% of HIV-infected patients in the post-antiretroviral era. We previously reported that two first generation HIV protease inhibitors, ritonavir and saquinavir, induced oxidative stress, with subsequent neuronal death in vitro, which was reversed by augmentation of the endogenous antioxidant response by monomethyl fumarate. We herein determined whether two newer-generation PIs, darunavir and lopinavir, were deleterious to neurons in vitro. Further, we expanded our assessment to include three integrase strand transfer inhibitors, raltegravir, dolutegravir, and elvitegravir. We found that only lopinavir and elvitegravir were neurotoxic to primary rat neuroglial cultures as determined by the loss of microtubule-associated protein 2 (MAP2). Intriguingly, lopinavir but not elvitegravir led to oxidative stress and induced the endogenous antioxidant response (EAR). Furthermore, neurotoxicity of lopinavir was blocked by pharmacological augmentation of the endogenous antioxidant heme oxygenase-1 (HO-1), expanding our previous finding that protease inhibitor-induced neurotoxicity was mediated by oxidative stress. Conversely, elvitegravir but not lopinavir led to increased eIF2α phosphorylation, indicating the activation of a common adaptive pathway termed the integrated stress response (ISR), and elvitegravir-mediated neurotoxicity was partially alleviated by the ISR inhibitor trans-ISRIB, suggesting ISR as a promoter of elvitegravir-associated neurotoxicity. Overall, we found that neurotoxicity was induced only by a subset of protease inhibitors and integrase strand transfer inhibitors, providing evidence for class- and drug-specific neurotoxic effects of antiretroviral drugs. Future in vivo studies will be critical to confirm the neurotoxicity profiles of these drugs for incorporation of these findings into patient management. The EAR and ISR pathways are potential access points for the development of adjunctive therapies to complement antiretroviral therapies and limit their contribution to HAND persistence.
Keywords: Antiretroviral therapy; HIV; HIV-associated neurocognitive disorders; Heme oxygenase-1; Integrated stress response; Oxidative stress.
Figures



References
-
- Akay C, Lindl KA, Shyam N, Nabet B, Goenaga-Vazquez Y, Ruzbarsky J, Wang Y, Kolson DL, Jordan-Sciutto KL. Activation status of integrated stress response pathways in neurones and astrocytes of HIV-associated neurocognitive disorders (HAND) cortex. Neuropathol Appl Neurobiol. 2012;38:175–200. - PMC - PubMed
-
- Anthonypillai C, Sanderson RN, Gibbs JE, Thomas SA. The distribution of the HIV protease inhibitor, ritonavir, to the brain, cerebrospinal fluid, and choroid plexuses of the Guinea pig. J Pharmacol Exp Ther. 2004;308:912–920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

