One-year result of XEN45 implant for glaucoma: efficacy, safety, and postoperative management
- PMID: 28862254
- PMCID: PMC5811690
- DOI: 10.1038/eye.2017.162
One-year result of XEN45 implant for glaucoma: efficacy, safety, and postoperative management
Abstract
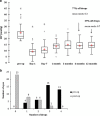
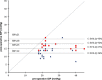
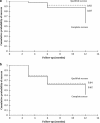
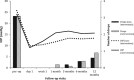
PurposeTo report the efficacy, safety profile, and postoperative management of XEN45 implant at 12-month follow-up.Patients and methodsThis was a retrospective, non-randomised interventional case series involving patients who underwent XEN45 implantation by a single, experienced glaucoma specialist in a tertiary centre. Primary outcome measures were the intraocular pressure (IOP) and the number of medications at 1-year follow-up visit. Two IOP criteria were chosen to measure success: IOP ≤21 mm Hg and ≥20% reduction from baseline (criteria one); and IOP ≤15 mm Hg and ≥30% reduction from baseline (criteria two).ResultsAll 39 eyes completed 1-year follow-up. The mean preoperative IOP was 24.9±7.8 mm Hg on three drops, which reduced to 14.5±3.4 mm Hg at month 12 (P<0.005) on 0.7 drops (P<0.005). On the basis of criteria one, the cumulative probability of success at 1 year was 87.0% without medication and 92.0% with medication. On the basis of criteria two, cumulative probability of success was 62.0% without medication and 64.0% with medication. Bleb intervention with a median of 2 (range 1-4) was required for 51.3% of eyes. Implant was obstructed by iris tissue in three eyes (7.7%); one eye (2.6%) developed hyphaema; eight eyes (20.5%) had numerical hypotony (IOP≤5 mm Hg) at day 1, of which all spontaneously resolved by week 4 apart from one eye.ConclusionsThe XEN45 implant proved to be an effective treatment with a good safety profile at 1-year follow-up period. The high rate of postoperative bleb intervention does not make XEN45 a 'fit-and-forget' procedure and therefore the procedure should ideally be performed by surgeons experienced in bleb management.
Conflict of interest statement
LA has received research support, honorarium, and travel reimbursement from Glaukos, Aquesys/Allergan, Ivantis, Eyetech care, Thea, and Alcon Pharmaceuticals. The remaining authors declare no conflict of interest.
Figures
References
-
- Craven ER, Katz LJ, Wells JM, Giamporcaro JE, Group iS. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg 2012; 38(8): 1339–1345. - PubMed
-
- Arriola-Villalobos P, Martínez-de-la-Casa JM, Díaz-Valle D, Fernández-Pérez C, García-Sánchez J, García-Feijoó J. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term study. Br J Ophthalmol 2012; 96(5): 645–649. - PubMed
-
- Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, Larrosa JM, Fea A, Lemij H et al. A randomized trial of a Schlemm's canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology 2015; 122(7): 1283–1293. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

