Understanding the functions and relationships of the glymphatic system and meningeal lymphatics
- PMID: 28862640
- PMCID: PMC5669566
- DOI: 10.1172/JCI90603
Understanding the functions and relationships of the glymphatic system and meningeal lymphatics
Abstract
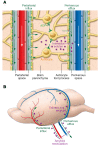
Recent discoveries of the glymphatic system and of meningeal lymphatic vessels have generated a lot of excitement, along with some degree of skepticism. Here, we summarize the state of the field and point out the gaps of knowledge that should be filled through further research. We discuss the glymphatic system as a system that allows CNS perfusion by the cerebrospinal fluid (CSF) and interstitial fluid (ISF). We also describe the recently characterized meningeal lymphatic vessels and their role in drainage of the brain ISF, CSF, CNS-derived molecules, and immune cells from the CNS and meninges to the peripheral (CNS-draining) lymph nodes. We speculate on the relationship between the two systems and their malfunction that may underlie some neurological diseases. Although much remains to be investigated, these new discoveries have changed our understanding of mechanisms underlying CNS immune privilege and CNS drainage. Future studies should explore the communications between the glymphatic system and meningeal lymphatics in CNS disorders and develop new therapeutic modalities targeting these systems.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

