Mepolizumab Attenuates Airway Eosinophil Numbers, but Not Their Functional Phenotype, in Asthma
- PMID: 28862877
- PMCID: PMC5736971
- DOI: 10.1164/rccm.201611-2234OC
Mepolizumab Attenuates Airway Eosinophil Numbers, but Not Their Functional Phenotype, in Asthma
Abstract
Rationale: Mepolizumab, an IL-5-blocking antibody, reduces exacerbations in patients with severe eosinophilic asthma. Mepolizumab arrests eosinophil maturation; however, the functional phenotype of eosinophils that persist in the blood and airway after administration of IL-5 neutralizing antibodies has not been reported.
Objectives: To determine the effect of anti-IL-5 antibody on the numbers and phenotypes of allergen-induced circulating and airway eosinophils.
Methods: Airway inflammation was elicited in participants with mild allergic asthma by segmental allergen challenge before and 1 month after a single intravenous 750-mg dose of mepolizumab. Eosinophils were examined in blood, bronchoalveolar lavage, and endobronchial biopsies 48 hours after challenge.
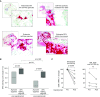
Measurements and main results: Segmental challenge without mepolizumab induced a rise in circulating eosinophils, bronchoalveolar lavage eosinophilia, and eosinophil peroxidase deposition in bronchial mucosa. IL-5 neutralization before allergen challenge abolished the allergen-induced rise in circulating eosinophils and expression of IL-3 receptors, whereas airway eosinophilia and eosinophil peroxidase deposition were blunted but not eliminated. Before mepolizumab treatment, bronchoalveolar lavage eosinophils had more surface IL-3 and granulocyte-monocyte colony-stimulating factor receptors, CD69, CD44, and CD23 and decreased IL-5 and eotaxin receptors than blood eosinophils. This activation phenotype indicated by bronchoalveolar lavage eosinophil surface markers, as well as the release of eosinophil peroxidase by eosinophils in the bronchial mucosa, was maintained after mepolizumab.
Conclusions: Mepolizumab reduced airway eosinophil numbers but had a limited effect on airway eosinophil activation markers, suggesting that these cells retain functionality. This observation may explain why IL-5 neutralization reduces but does not completely eradicate asthma exacerbations. Clinical trial registered with www.clinicaltrials.gov (NCT00802438).
Keywords: IL-3 receptor; IL-5; allergen challenge.
Figures





Comment in
-
Pauca sed Matura: Few, but Ripe.Am J Respir Crit Care Med. 2017 Dec 1;196(11):1361-1362. doi: 10.1164/rccm.201709-1848ED. Am J Respir Crit Care Med. 2017. PMID: 28926280 No abstract available.
References
-
- Volbeda F, Broekema M, Lodewijk ME, Hylkema MN, Reddel HK, Timens W, Postma DS, ten Hacken NH. Clinical control of asthma associates with measures of airway inflammation. Thorax. 2013;68:19–24. - PubMed
-
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–1721. - PubMed
-
- Smith DA, Minthorn EA, Beerahee M. Pharmacokinetics and pharmacodynamics of mepolizumab, an anti–interleukin-5 monoclonal antibody. Clin Pharmacokinet. 2011;50:215–227. - PubMed
-
- Keating GM. Mepolizumab: first global approval. Drugs. 2015;75:2163–2169. - PubMed
-
- Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID SIRIUS Investigators. Oral glucocorticoid–sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

