The novel Lyme borreliosis vaccine VLA15 shows broad protection against Borrelia species expressing six different OspA serotypes
- PMID: 28863166
- PMCID: PMC5581183
- DOI: 10.1371/journal.pone.0184357
The novel Lyme borreliosis vaccine VLA15 shows broad protection against Borrelia species expressing six different OspA serotypes
Abstract
We have previously shown that the Outer surface protein A (OspA) based Lyme borreliosis vaccine VLA15 induces protective immunity in mice. Herein, we report the induction of protective immunity by VLA15 with mouse models using ticks infected with B. burgdorferi (OspA serotype 1), B. afzelii (OspA serotype 2) and B. bavariensis (OspA serotype 4) or with in vitro grown B. garinii (OspA serotype 5 and 6) for challenge. For B. garinii (OspA serotype 3), we have developed a growth inhibition assay using chicken complement and functional antibodies targeting B. garinii (OspA serotype 3) could be demonstrated after immunization with VLA15. Furthermore, following three priming immunizations, a booster dose was administered five months later and the induction of immunological memory could be confirmed. Thus, the antibody titers after the booster dose were increased considerably compared to those after primary immunization. In addition, the half-lives of anti-OspA serotype specific antibodies after administration of the booster immunization were longer than after primary immunization. Taken together, we could show that VLA15 induced protection in mice against challenge with four different clinically relevant Borrelia species (B. burgdorferi, B. afzelii, B. garinii and B. bavariensis) expressing five of the six OspA serotypes included in the vaccine. The protection data is supported by functional assays showing efficacy against spirochetes expressing any of the six OspA serotypes (1 to 6). To our knowledge, this is the first time a Lyme borreliosis vaccine has been able to demonstrate such broad protection in preclinical studies. These new data provide further promise for the clinical development of VLA15 and supports our efforts to provide a new Lyme borreliosis vaccine available for global use.
Conflict of interest statement
Figures
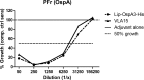
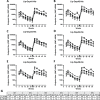
References
-
- Sigal LH, Zahradnik JM, Lavin P, Patella SJ, Bryant G, Haselby R, et al. (1998) A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. Recombinant Outer-Surface Protein A Lyme Disease Vaccine Study Consortium. N Engl J Med 339: 216–222. doi: 10.1056/NEJM199807233390402 - DOI - PubMed
-
- Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, et al. (1998) Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N Engl J Med 339: 209–215. doi: 10.1056/NEJM199807233390401 - DOI - PubMed
-
- Wressnigg N, Pöllabauer EM, Aichinger G, Portsmouth D, Low-Baselli A, Fritsch S, et al. (2013) Safety and immunogenicity of a novel multivalent OspA vaccine against Lyme borreliosis in healthy adults: a double-blind, randomised, dose-escalation phase 1/2 trial. Lancet Infect Dis 13: 680–689. doi: 10.1016/S1473-3099(13)70110-5 - DOI - PubMed
-
- Poland GA (2011) Vaccines against Lyme disease: What happened and what lessons can we learn? Clin Infect Dis 52 Suppl 3: s253–258. - PubMed
-
- Nigrovic LE, Thompson KM (2007) The Lyme vaccine: a cautionary tale. Epidemiol Infect 135: 1–8. doi: 10.1017/S0950268806007096 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

