Characterization of 2 Novel Ependymoma Cell Lines With Chromosome 1q Gain Derived From Posterior Fossa Tumors of Childhood
- PMID: 28863455
- PMCID: PMC5868094
- DOI: 10.1093/jnen/nlx040
Characterization of 2 Novel Ependymoma Cell Lines With Chromosome 1q Gain Derived From Posterior Fossa Tumors of Childhood
Abstract
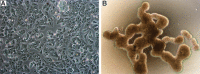
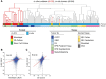
Ependymoma (EPN) is a common brain tumor of childhood that, despite standard surgery and radiation therapy, has a relapse rate of 50%. Clinical trials have been unsuccessful in improving outcome by addition of chemotherapy, and identification of novel therapeutics has been hampered by a lack of in vitro and in vivo models. We describe 2 unique EPN cell lines (811 and 928) derived from recurrent intracranial metastases. Both cell lines harbor the high-risk chromosome 1q gain (1q+) and a derivative chromosome 6, and both are classified as molecular group A according to transcriptomic analysis. Transcriptional enrichment of extracellular matrix-related genes was a common signature of corresponding primary tumors and cell lines in both monolayer and 3D formats. EPN cell lines, when cultured in 3D format, clustered closer to the primary tumors with better fidelity of EPN-specific transcripts than when grown as a monolayer. Additionally, 3D culture revealed ependymal rosette formation and cilia-related ontologies, similar to in situ tumors. Our data confirm the validity of the 811 and 928 cell lines as representative models of intracranial, posterior fossa 1q+ EPN, which holds potential to advance translational science for patients affected by this tumor.
Keywords: Cell line; Chromosome 1q gain; Ependymoma.
© 2017 American Association of Neuropathologists, Inc. All rights reserved.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

