Treatment-Induced Viral Cure of Hepatitis C Virus-Infected Patients Involves a Dynamic Interplay among three Important Molecular Players in Lipid Homeostasis: Circulating microRNA (miR)-24, miR-223, and Proprotein Convertase Subtilisin/Kexin Type 9
- PMID: 28864162
- PMCID: PMC5605363
- DOI: 10.1016/j.ebiom.2017.08.020
Treatment-Induced Viral Cure of Hepatitis C Virus-Infected Patients Involves a Dynamic Interplay among three Important Molecular Players in Lipid Homeostasis: Circulating microRNA (miR)-24, miR-223, and Proprotein Convertase Subtilisin/Kexin Type 9
Abstract
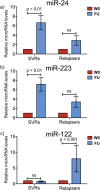
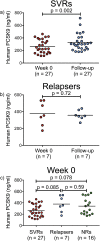
In patients with chronic hepatitis C virus (HCV) infection, viral hijacking of the host-cell biosynthetic pathways is associated with altered lipid metabolism, which contributes to disease progression and may influence antiviral response. We investigated the molecular interplay among four key regulators of lipid homeostasis [microRNA (miR)-122, miR-24, miR-223, and proprotein convertase subtilisin/kexin type 9 (PCSK9)] in HCV-infected patients (n=72) who achieved a treatment-based viral cure after interferon-based therapy with first-generation direct-acting antivirals. Real-time PCR was used to quantify microRNA plasma levels, and ELISA assays were used to determine plasma concentrations of PCSK9. We report that levels of miR-24 and miR-223 significantly increased in patients achieving sustained virologic response (SVR), whereas the levels of miR-122, a liver-specific cofactor for HCV infection, decreased in these patients. PCSK9 concentrations were significantly increased in SVRs, suggesting that PCSK9 may help impede viral infection. The modulatory effect of PCSK9 on HCV infection was also demonstrated in the context of HCV-infected Huh-7.5.1 cells employing recombinant human PCSK9 mutants. Together, these results provide insights into a novel coordinated interplay among three important molecular players in lipid homeostasis - circulating miR-24, miR-223 and PCSK9 - whose regulation is affected by HCV infection and treatment-based viral cure.
Keywords: Antiviral therapy; Hepatitis C virus; Proprotein convertase subtilisin/kexin type 9; miR-122; miR-223; miR-24.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Hepatitis C virus and proprotein convertase subtilisin/kexin type 9: a detrimental interaction to increase viral infectivity and disrupt lipid metabolism.J Cell Mol Med. 2017 Dec;21(12):3150-3161. doi: 10.1111/jcmm.13273. Epub 2017 Jul 18. J Cell Mol Med. 2017. PMID: 28722331 Free PMC article. Review.
-
PCSK9, apolipoprotein E and lipoviral particles in chronic hepatitis C genotype 3: evidence for genotype-specific regulation of lipoprotein metabolism.J Hepatol. 2015 Apr;62(4):763-70. doi: 10.1016/j.jhep.2014.11.016. Epub 2014 Nov 21. J Hepatol. 2015. PMID: 25463543
-
Increased hepatic expression of miRNA-122 in patients infected with HCV genotype 3.Med Microbiol Immunol. 2016 Apr;205(2):111-7. doi: 10.1007/s00430-015-0431-0. Epub 2015 Aug 14. Med Microbiol Immunol. 2016. PMID: 26272127
-
Proprotein convertase subtilisin/kexin type 9 inhibits hepatitis C virus replication through interacting with NS5A.J Gen Virol. 2018 Jan;99(1):44-61. doi: 10.1099/jgv.0.000987. Epub 2017 Dec 13. J Gen Virol. 2018. PMID: 29235977
-
Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis.J Am Coll Cardiol. 2013 Oct 15;62(16):1401-8. doi: 10.1016/j.jacc.2013.07.056. Epub 2013 Aug 21. J Am Coll Cardiol. 2013. PMID: 23973703 Review.
Cited by
-
Role of miR-223 in the pathophysiology of liver diseases.Exp Mol Med. 2018 Sep 26;50(9):1-12. doi: 10.1038/s12276-018-0153-7. Exp Mol Med. 2018. PMID: 30258086 Free PMC article. Review.
-
MicroRNA-770 affects proliferation and cell cycle transition by directly targeting CDK8 in glioma.Cancer Cell Int. 2018 Dec 3;18:195. doi: 10.1186/s12935-018-0694-9. eCollection 2018. Cancer Cell Int. 2018. PMID: 30524203 Free PMC article.
-
MiR-223 as a Regulator and Therapeutic Target in Liver Diseases.Front Immunol. 2022 Mar 16;13:860661. doi: 10.3389/fimmu.2022.860661. eCollection 2022. Front Immunol. 2022. PMID: 35371024 Free PMC article. Review.
-
Reprogramming of cellular metabolic pathways by human oncogenic viruses.Curr Opin Virol. 2019 Dec;39:60-69. doi: 10.1016/j.coviro.2019.11.002. Epub 2019 Nov 22. Curr Opin Virol. 2019. PMID: 31766001 Free PMC article. Review.
-
Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics.Signal Transduct Target Ther. 2022 Aug 2;7(1):265. doi: 10.1038/s41392-022-01125-5. Signal Transduct Target Ther. 2022. PMID: 35918332 Free PMC article. Review.
References
-
- Abifadel M., Varret M., Rabes J.P., Allard D., Ouguerram K., Devillers M., Cruaud C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003;34:154–156. - PubMed
-
- Alborn W.E., Cao G., Careskey H.E., Qian Y.W., Subramaniam D.R., Davies J., Conner E.M. Serum proprotein convertase subtilisin kexin type 9 is correlated directly with serum LDL cholesterol. Clin. Chem. 2007;53:1814–1819. - PubMed
-
- Bassendine M.F., Sheridan D.A., Bridge S.H., Felmlee D.J., Neely R.D. Lipids and HCV. Semin. Immunopathol. 2013;35:87–100. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

