Common evidence gaps in point-of-care diagnostic test evaluation: a review of horizon scan reports
- PMID: 28864692
- PMCID: PMC5588931
- DOI: 10.1136/bmjopen-2016-015760
Common evidence gaps in point-of-care diagnostic test evaluation: a review of horizon scan reports
Abstract
Objective: Since 2008, the Oxford Diagnostic Horizon Scan Programme has been identifying and summarising evidence on new and emerging diagnostic technologies relevant to primary care. We used these reports to determine the sequence and timing of evidence for new point-of-care diagnostic tests and to identify common evidence gaps in this process.
Design: Systematic overview of diagnostic horizon scan reports.
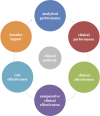
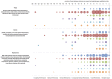
Primary outcome measures: We obtained the primary studies referenced in each horizon scan report (n=40) and extracted details of the study size, clinical setting and design characteristics. In particular, we assessed whether each study evaluated test accuracy, test impact or cost-effectiveness. The evidence for each point-of-care test was mapped against the Horvath framework for diagnostic test evaluation.
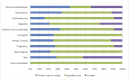
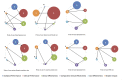
Results: We extracted data from 500 primary studies. Most diagnostic technologies underwent clinical performance (ie, ability to detect a clinical condition) assessment (71.2%), with very few progressing to comparative clinical effectiveness (10.0%) and a cost-effectiveness evaluation (8.6%), even in the more established and frequently reported clinical domains, such as cardiovascular disease. The median time to complete an evaluation cycle was 9 years (IQR 5.5-12.5 years). The sequence of evidence generation was typically haphazard and some diagnostic tests appear to be implemented in routine care without completing essential evaluation stages such as clinical effectiveness.
Conclusions: Evidence generation for new point-of-care diagnostic tests is slow and tends to focus on accuracy, and overlooks other test attributes such as impact, implementation and cost-effectiveness. Evaluation of this dynamic cycle and feeding back data from clinical effectiveness to refine analytical and clinical performance are key to improve the efficiency of point-of-care diagnostic test development and impact on clinically relevant outcomes. While the 'road map' for the steps needed to generate evidence are reasonably well delineated, we provide evidence on the complexity, length and variability of the actual process that many diagnostic technologies undergo.
Keywords: Diagnosis; PRIMARY CARE; Point-of-care Systems; evidence based medicine; framework; horizon scanning reports.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: MJT has received funding from Alere to conduct research and has provided consultancy to Roche Molecular Diagnostics. He is also a co-founder of Phoresia which is developing point-of-care tests. All the other authors have no competing interests to disclose.
Figures

References
-
- GV Research IVD. Market Analysis By Product (Tissue Diagnostics, Molecular Diagnostics, Professional Diagnostics, Diabetes Monitoring) And Segment Forecasts To 2020. 100 Pune, India: Grand View Research, 2016.
-
- Greenhalgh T, Robert G, Bate P, et al. How to spread good ideas: a systematic review of the literature on diffusion, dissemination and sustainability of innovations in health service delivery and organisation. London: NCCSDO, 2004.
-
- Britain G, National Health S. The NHS atlas of variation in diagnostic services: reducing unwarranted variation to increase value and improve quality. Great Britain: Right Care, 2013.
-
- Oxford DEC. NIHR Diagnostic evidence Cooperative Oxford. 2016. https://www.oxford.dec.nihr.ac.uk/
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
