Does supplementation with carnosine improve cardiometabolic health and cognitive function in patients with pre-diabetes and type 2 diabetes? study protocol for a randomised, double-blind, placebo-controlled trial
- PMID: 28864708
- PMCID: PMC5588946
- DOI: 10.1136/bmjopen-2017-017691
Does supplementation with carnosine improve cardiometabolic health and cognitive function in patients with pre-diabetes and type 2 diabetes? study protocol for a randomised, double-blind, placebo-controlled trial
Abstract
Introduction: Carnosine, an over-the-counter food supplement, has a promising potential for the prevention and treatment of chronic diseases such as type 2 diabetes (T2DM), cardiovascular and neurodegenerative diseases through its anti-inflammatory, antiglycation, antioxidative and chelating effects. We have previously shown that supplementation with carnosine preserves insulin sensitivity and secretion in non-diabetic overweight and obese individuals. The effect of carnosine on cardiometabolic risk and related cognitive outcomes in patients with pre-diabetes and T2DM has thus far not been studied. We therefore aim to investigate whether supplementation with carnosine improves cardiometabolic health and cognitive function in patients with pre-diabetes and T2DM.
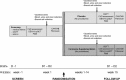
Methods and analysis: We will employ a parallel design randomised controlled trial. Fifty participants with pre-diabetes (impaired fasting glycaemia and impaired glucose tolerance) and T2DM (with HbA1c level < 8%) aged between 18 to 70 years will be randomly assigned to the intervention or control group. At baseline, participants will undergo a medical review and series of tests including anthropometric measurements (body mass index, a dual X-ray absorptiometry and peripheral quantitative computed tomography scan), an oral glucose tolerance test, cardiovascular measurements (central blood pressure, endothelial function and arterial stiffness), cognitive function, physical activity measurement, heart rate variability and liver fibroscan as well as questionnaires to assess dietary habits, sleep quality, depression and quality of life. The intervention group will receive 2 g of carnosine daily in two divided doses while the control group will receive identical placebo capsules for 14 weeks. All baseline measurements will be repeated at the end of the intervention. The change in glycaemic, cardiovascular and cognitive parameters as well as other measures will be compared between the groups.
Ethics and dissemination: This study is approved by the Human Research Ethics Committee of Monash Health and Monash University, Australia. The findings will be disseminated via peer-reviewed publications and conference presentations.
Trial registration: NCT02917928; Pre-results.
Keywords: general diabetes; lipid disorders; other metabolic, e.g. iron, porphyria.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Danaei G, Finucane MM, Lu Y, et al. . National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011;378:31–40.10.1016/S0140-6736(11)60679-X - DOI - PubMed
-
- IDF. Diabetes Atlas Sixth Edition Update: international Diabetes Federation, 2014.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical