Immuno-oncology Clinical Trial Design: Limitations, Challenges, and Opportunities
- PMID: 28864727
- PMCID: PMC5735832
- DOI: 10.1158/1078-0432.CCR-16-3066
Immuno-oncology Clinical Trial Design: Limitations, Challenges, and Opportunities
Abstract
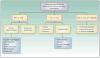
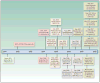
Recent advances in immuno-oncology and regulatory approvals have been rapid and paradigm shifting in many difficult-to-treat malignancies. Despite immune checkpoint inhibitor therapy becoming the standard of care across multiple tumor types, there are many unanswered questions that need to be addressed before this therapeutic modality can be fully harnessed. Areas of limitations include treatment of patients not sufficiently represented in clinical trials, uncertainty of the optimal treatment dosing and duration, and lack of understanding regarding long-term immune related toxicities and atypical tumor responses. Patients such as those with autoimmune disease, chronic viral infections, limited performance status, and brain metastases were often excluded from initial trials due to concerns of safety. However, limited data suggest that some of these patients can benefit from therapy with manageable toxicities; thus, future studies should incorporate these patients to clearly define safety and efficacy. There are still controversies regarding the optimal dosing strategy that can vary from weight-based to flat dosing, with undefined treatment duration. Further elucidation of the optimal dosing approach and evaluation of predictive biomarkers should be incorporated in the design of future trials. Finally, there are long-term immune-mediated toxicities, atypical tumor responses such as pseudoprogression and endpoints unique to immuno-oncology that are not adequately captured by traditional trial designs; thus, novel study designs are needed. In this article, we discuss in detail the above challenges and propose needed areas of research for exploration and incorporation in the next generation of immuno-oncology clinical trials.
©2017 American Association for Cancer Research.
Conflict of interest statement
C.S. Baik is a consultant/advisory board member for Novartis. P.M. Forde is a consultant/advisory board member for AstraZeneca, BMS, Boehringer, Celgene, EMD Serono, Merck, and Novartis. J.M. Mehnert reports receiving other commercial research support from AstraZeneca, EMD Serono, Incyte, Macrogenics, and Merck and is a consultant/advisory board member for EMD Serono, Genentech, Merck, and Pfizer. M.O. Butler reports receiving commercial research grants from Merck and is a consultant/advisory board member for Bristol-Myers Squibb, EMD Serono, Immunocore, Immunovaccine, Merck, Novartis, and Turnstone. L.Q.M. Chow is a consultant/advisory board member for Amgen, AstraZeneca/Medimmune, Bristol-Myers Squibb, Genentech, Merck, Novartis, Pfizer, and Seattle Genetics. No potential conflicts of interest were disclosed by the other authors.
Figures
References
-
- Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2016;373:mdw443. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

