Negatively charged residues in the first extracellular loop of the L-type CaV1.2 channel anchor the interaction with the CaVα2δ1 auxiliary subunit
- PMID: 28864774
- PMCID: PMC5655503
- DOI: 10.1074/jbc.M117.806893
Negatively charged residues in the first extracellular loop of the L-type CaV1.2 channel anchor the interaction with the CaVα2δ1 auxiliary subunit
Abstract
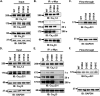
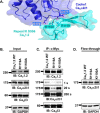
Voltage-gated L-type CaV1.2 channels in cardiomyocytes exist as heteromeric complexes. Co-expression of CaVα2δ1 with CaVβ/CaVα1 proteins reconstitutes the functional properties of native L-type currents, but the interacting domains at the CaV1.2/CaVα2δ1 interface are unknown. Here, a homology-based model of CaV1.2 identified protein interfaces between the extracellular domain of CaVα2δ1 and the extracellular loops of the CaVα1 protein in repeats I (IS1S2 and IS5S6), II (IIS5S6), and III (IIIS5S6). Insertion of a 9-residue hemagglutinin epitope in IS1S2, but not in IS5S6 or in IIS5S6, prevented the co-immunoprecipitation of CaV1.2 with CaVα2δ1. IS1S2 contains a cluster of three conserved negatively charged residues Glu-179, Asp-180, and Asp-181 that could contribute to non-bonded interactions with CaVα2δ1. Substitutions of CaV1.2 Asp-181 impaired the co-immunoprecipitation of CaVβ/CaV1.2 with CaVα2δ1 and the CaVα2δ1-dependent shift in voltage-dependent activation gating. In contrast, single substitutions in CaV1.2 in neighboring positions in the same loop (179, 180, and 182-184) did not significantly alter the functional up-regulation of CaV1.2 whole-cell currents. However, a negatively charged residue at position 180 was necessary to convey the CaVα2δ1-mediated shift in the activation gating. We also found a more modest contribution from the positively charged Arg-1119 in the extracellular pore region in repeat III of CaV1.2. We conclude that CaV1.2 Asp-181 anchors the physical interaction that facilitates the CaVα2δ1-mediated functional modulation of CaV1.2 currents. By stabilizing the first extracellular loop of CaV1.2, CaVα2δ1 may up-regulate currents by promoting conformations of the voltage sensor that are associated with the channel's open state.
Keywords: calcium channel; electrophysiology; gating; molecular modeling; protein-protein interaction.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures







References
-
- Bers D. M. (2000) Calcium fluxes involved in control of cardiac myocyte contraction. Circ. Res. 87, 275–281 - PubMed
-
- Jaleel N., Nakayama H., Chen X., Kubo H., MacDonnell S., Zhang H., Berretta R., Robbins J., Cribbs L., Molkentin J. D., and Houser S. R. (2008) Calcium influx through T- and L-type calcium channels have different effects on myocyte contractility and induce unique cardiac phenotypes. Circ. Res. 103, 1109–1119 - PMC - PubMed
-
- Gao H., Wang F., Wang W., Makarewich C. A., Zhang H., Kubo H., Berretta R. M., Barr L. A., Molkentin J. D., and Houser S. R. (2012) Calcium influx through L-type calcium channels and transient receptor potential channels activates pathological hypertrophy signaling. J. Mol. Cell. Cardiol. 53, 657–667 - PMC - PubMed
-
- Bannister J. P., Bulley S., Narayanan D., Thomas-Gatewood C., Luzny P., Pachuau J., and Jaggar J. H. (2012) Transcriptional upregulation of α2δ1 elevates arterial smooth muscle cell voltage-dependent Ca2+ channel surface expression and cerebrovascular constriction in genetic hypertension. Hypertension 60, 1006–1015 - PMC - PubMed
-
- Yue L., Feng J., Gaspo R., Li G. R., Wang Z., and Nattel S. (1997) Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ. Res. 81, 512–525 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

