Eltrombopag: a powerful chelator of cellular or extracellular iron(III) alone or combined with a second chelator
- PMID: 28864815
- PMCID: PMC6558288
- DOI: 10.1182/blood-2016-10-740241
Eltrombopag: a powerful chelator of cellular or extracellular iron(III) alone or combined with a second chelator
Abstract
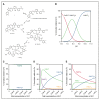
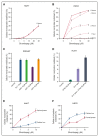
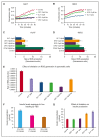
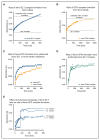
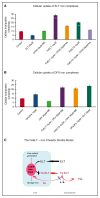
Eltrombopag (ELT) is a thrombopoietin receptor agonist reported to decrease labile iron in leukemia cells. Here we examine the previously undescribed iron(III)-coordinating and cellular iron-mobilizing properties of ELT. We find a high binding constant for iron(III) (log β2=35). Clinically achievable concentrations (1 µM) progressively mobilized cellular iron from hepatocyte, cardiomyocyte, and pancreatic cell lines, rapidly decreasing intracellular reactive oxygen species (ROS) and also restoring insulin secretion in pancreatic cells. Decrements in cellular ferritin paralleled total cellular iron removal, particularly in hepatocytes. Iron mobilization from cardiomyocytes exceeded that obtained with deferiprone, desferrioxamine, or deferasirox at similar iron-binding equivalents. When combined with these chelators, ELT enhanced cellular iron mobilization more than additive (synergistic) with deferasirox. Iron-binding speciation plots are consistent with ELT donating iron to deferasirox at clinically relevant concentrations. ELT scavenges iron citrate species faster than deferasirox, but rapidly donates the chelated iron to deferasirox, consistent with a shuttling mechanism. Shuttling is also suggested by enhanced cellular iron mobilization by ELT when combined with the otherwise ineffective extracellular hydroxypyridinone chelator, CP40. We conclude that ELT is a powerful iron chelator that decreases cellular iron and further enhances iron mobilization when combined with clinically available chelators.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict of interest disclosure: The authors declare no competing financial interests.
Figures
References
-
- Williams DD, Peng B, Bailey CK, et al. Effects of food and antacids on the pharmacokinetics of eltrombopag in healthy adult subjects: two single-dose, open-label, randomized-sequence, crossover studies. Clin Ther. 2009;31(4):764–776. - PubMed
-
- Wire MB, Bruce J, Gauvin J, et al. A randomized, open-label, 5-period, balanced crossover study to evaluate the relative bioavailability of eltrombopag powder for oral suspension (PfOS) and tablet formulations and the effect of a high-calcium meal on eltrombopag pharmacokinetics when administered with or 2 hours before or after PfOS. Clin Ther. 2012;34(3):699–709. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

