Oxytocin Modulation of Neural Circuits
- PMID: 28864972
- PMCID: PMC5834368
- DOI: 10.1007/7854_2017_7
Oxytocin Modulation of Neural Circuits
Abstract
Oxytocin is a hypothalamic neuropeptide first recognized as a regulator of parturition and lactation which has recently gained attention for its ability to modulate social behaviors. In this chapter, we review several aspects of the oxytocinergic system, focusing on evidence for release of oxytocin and its receptor distribution in the cortex as the foundation for important networks that control social behavior. We examine the developmental timeline of the cortical oxytocin system as demonstrated by RNA, autoradiographic binding, and protein immunohistochemical studies, and describe how that might shape brain development and behavior. Many recent studies have implicated oxytocin in cognitive processes such as processing of sensory stimuli, social recognition, social memory, and fear. We review these studies and discuss the function of oxytocin in the young and adult cortex as a neuromodulator of central synaptic transmission and mediator of plasticity.
Keywords: Cortex; Inhibition; Neuromodulation; Oxytocin; Synaptic plasticity.
Figures
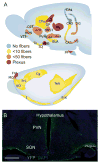
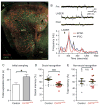

References
-
- Bales KL, Carter CS. Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2003a;117(4):854–859. - PubMed
-
- Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm Behav. 2003b;44(3):178–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

