Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 2 hepatitis C virus infection
- PMID: 28865152
- PMCID: PMC5814891
- DOI: 10.1002/hep.29510
Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 2 hepatitis C virus infection
Abstract
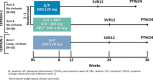
Glecaprevir (nonstructural protein 3/4A protease inhibitor) and pibrentasvir (nonstructural protein 5A inhibitor) (G/P), a coformulated once-daily, all oral, ribavirin (RBV)-free, direct-acting antiviral regimen, was evaluated for safety and efficacy in hepatitis C virus genotype 2 (GT2)-infected Japanese patients, including those with compensated cirrhosis. CERTAIN-2 is a phase 3, open-label, multicenter study assessing the safety and efficacy of G/P (300/120 mg) once daily in treatment-naive and interferon ± RBV treatment-experienced Japanese patients without cirrhosis but with GT2 infection. Patients were randomized 2:1 to receive 8 weeks of G/P (arm A) or 12 weeks of sofosbuvir (400 mg once daily) + RBV (600-1000 mg weight-based, twice daily) (arm B). The primary endpoint was noninferiority of G/P compared to sofosbuvir + RBV by assessing sustained virologic response at posttreatment week 12 (SVR12) among patients in the intent-to-treat population. SVR12 was also assessed in treatment-naive and interferon ± RBV treatment-experienced patients with GT2 infection and compensated cirrhosis who received G/P for 12 weeks in the CERTAIN-1 study. A total of 136 patients were enrolled in CERTAIN-2. SVR12 was achieved by 88/90 (97.8%) patients in arm A and 43/46 (93.5%) patients in arm B. No patient in arm A experienced virologic failure, while 2 did in arm B. The primary endpoint was achieved. In CERTAIN-1, 100% (18/18) of GT2-infected patients with compensated cirrhosis achieved SVR12. Treatment-emergent serious adverse events were experienced by 2 patients without cirrhosis in each arm and no patient with cirrhosis. Conclusion: The results demonstrate high efficacy and favorable tolerability of G/P in GT2-infected Japanese patients. (Hepatology 2018;67:505-513).
Trial registration: ClinicalTrials.gov NCT02723084 NCT02707952.
© 2017 The Authors. Hepatology published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Figures

References
-
- Bennett H, Waser N, Johnston K, Kao JH, Lim YS, Duan ZP, et al. A review of the burden of hepatitis C virus infection in China, Japan, South Korea and Taiwan. Hepatol Int 2015;9:378‐390. - PubMed
-
- Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, Alomair A, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int 2011;31(Suppl. 2):61‐80. - PubMed
-
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age‐specific antibody to HCV seroprevalence. Hepatology 2013;57:1333‐1342. - PubMed
-
- Liu GG, DiBonaventura M, Yuan Y, Wagner JS, L'Italien GJ, Langley P, et al. The burden of illness for patients with viral hepatitis C: evidence from a national survey in Japan. Value Health 2012;15:S65‐S71. - PubMed
-
- Yu ML, Chuang WL. Treatment of chronic hepatitis C in Asia: when East meets West. J Gastroenterol Hepatol 2009;24:336‐345. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

