Principles of pharmacology in the eye
- PMID: 28865239
- PMCID: PMC5715579
- DOI: 10.1111/bph.14024
Principles of pharmacology in the eye
Abstract
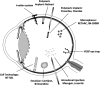
The eye is a highly specialized organ that is subject to a huge range of pathology. Both local and systemic disease may affect different anatomical regions of the eye. The least invasive routes for ocular drug administration are topical (e.g. eye drops) and systemic (e.g. tablets) formulations. Barriers that subserve as protection against pathogen entry also restrict drug permeation. Topically administered drugs often display limited bioavailability due to many physical and biochemical barriers including the pre-corneal tear film, the structure and biophysiological properties of the cornea, the limited volume that can be accommodated by the cul-de-sac, the lacrimal drainage system and reflex tearing. The tissue layers of the cornea and conjunctiva are further key factors that act to restrict drug delivery. Using carriers that enhance viscosity or bind to the ocular surface increases bioavailability. Matching the pH and polarity of drug molecules to the tissue layers allows greater penetration. Drug delivery to the posterior segment is a greater challenge and, currently, the standard route is via intravitreal injection, notwithstanding the risks of endophthalmitis and retinal detachment with frequent injections. Intraocular implants that allow sustained drug release are at different stages of development. Novel exciting therapeutic approaches include methods for promoting transscleral delivery, sustained release devices, nanotechnology and gene therapy.
© 2017 The British Pharmacological Society.
Figures
References
-
- Ahmed I, Patton TF (1985). Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci 26: 584–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical