Investigating the state of physiologically based kinetic modelling practices and challenges associated with gaining regulatory acceptance of model applications
- PMID: 28866268
- PMCID: PMC5656087
- DOI: 10.1016/j.yrtph.2017.08.019
Investigating the state of physiologically based kinetic modelling practices and challenges associated with gaining regulatory acceptance of model applications
Abstract
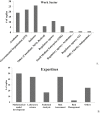
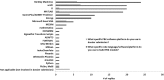
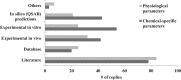
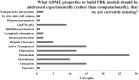
Physiologically based kinetic (PBK) models are used widely throughout a number of working sectors, including academia and industry, to provide insight into the dosimetry related to observed adverse health effects in humans and other species. Use of these models has increased over the last several decades, especially in conjunction with emerging alternative methods to animal testing, such as in vitro studies and data-driven in silico quantitative-structure-activity-relationship (QSAR) predictions. Experimental information derived from these new approach methods can be used as input for model parameters and allows for increased confidence in models for chemicals that did not have in vivo data for model calibration. Despite significant advancements in good modelling practice (GMP) for model development and evaluation, there remains some reluctance among regulatory agencies to use such models during the risk assessment process. Here, the results of a survey disseminated to the modelling community are presented in order to inform the frequency of use and applications of PBK models in science and regulatory submission. Additionally, the survey was designed to identify a network of investigators involved in PBK modelling and knowledgeable of GMP so that they might be contacted in the future for peer review of PBK models, especially in regards to vetting the models to such a degree as to gain a greater acceptance for regulatory purposes.
Keywords: Alternatives methods; PBK models; Physiologically based kinetic models; Regulatory; Risk assessment; Toxicology.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Ankley G.T., Bennett R.S., Erickson R.J., Hoff D.J., Hornung M.W., Johnson R.D., Mount D.R., Nichols J.W., Russom C.L., Schmieder P.K., Serrrano J.A., Tietge J.E., Villeneuve D.L. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010 Mar;29(3):730–741. - PubMed
-
- Bessems J.G., Loizou G., Krishnan K., Clewell H.J., 3rd, Bernasconi C., Bois F., Coecke S., Collnot E.M., Diembeck W., Farcal L.R., Geraets L., Gundert-Remy U., Kramer N., Küsters G., Leite S.B., Pelkonen O.R., Schröder K., Testai E., Wilk-Zasadna I., Zaldívar-Comenges J.M. PBTK modelling platforms and parameter estimation tools to enable animal-free risk assessment: recommendations from a joint EPAA–EURL ECVAM ADME workshop. Regul. Toxicol. Pharmacol. 2014 Feb;68(1):119–139. Epub 2013 Nov 26. - PubMed
-
- Bessems J.G.M., Paini A., Gajewska M., Worth A. 2017. The Margin of Internal Exposure (MOIE) Concept for Dermal Risk Assessment Based on Oral Toxicity Data – a Case Study with Caffeine, Toxicology.http://doi.org/10.1016/j.tox.2017.03.012 Available online 10 March 2017, ISSN 0300-483X. - DOI - PMC - PubMed
-
- Bhat V.S., Meek M.E.B., Valcke M., English C., Boobis A., Brown R. Evolution of chemical-specific adjustment factors (CSAF) based on recent international experience; increasing utility and facilitating regulatory acceptance. Crit. Rev. Toxicol. 2017 - PubMed
-
- Binetti R., Costamagna F.M., Marcello I. Exponential growth of new chemicals and evolution of information relevant to risk control. Ann. Ist. Super. Sanita. 2008;44:13–15. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

