SAVE-AMD: Safety of VEGF Inhibitors in Age-Related Macular Degeneration
- PMID: 28866675
- PMCID: PMC5804860
- DOI: 10.1159/000478665
SAVE-AMD: Safety of VEGF Inhibitors in Age-Related Macular Degeneration
Abstract
Objective: To determine whether intraocular treatment with vascular endothelial growth factor (VEGF) inhibitors change systemic endothelial function (EF) in patients with neovascular age-related macular degeneration (AMD).
Methods: In this prospective, randomized, 2-center, double-masked controlled interventional trial, patients with neovascular and dry AMD were enrolled. Eligible neovascular AMD patients received 2 intravitreal loading doses of either ranibizumab 0.5 mg or bevacizumab 1.25 mg at 4-week intervals and were subsequently followed every 4 weeks and treated according to a pro re nata regime for up to 1 year. Patients with dry AMD served as controls. The primary endpoint was the change in EF assessed by flow-mediated dilatation (FMD) after 2 months of treatment with VEGF inhibitors in patients with AMD compared to patients with dry AMD. FMD was assessed with B-mode high-resolution ultrasonography of the left brachial artery.
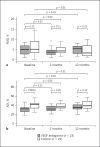
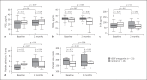
Results: 24 patients with neovascular AMD and 26 patients with dry ADM were included in the trial. Treatment with VEGF inhibitors did not significantly change FMD (from 4.7 ± 2.4 to 3.9 ± 1.9% after 8 weeks, p = 0.07, and to 5.1 ± 2.0% after 1 year; p = 0.93 vs. baseline, respectively).
Conclusions: EF did not significantly differ between patients with neovascular AMD treated with intravitreal VEGF inhibition and patients with dry AMD.
Keywords: Age-related macular degeneration; Bevacizumab; Clinical trial; Endothelial function; Ranibizumab; Vascular endothelial growth factor.
© 2017 The Author(s) Published by S. Karger AG, Basel.
Figures
References
-
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. - PubMed
-
- Heier JS, Boyer DS, Ciulla TA, Ferrone PJ, Jumper JM, Gentile RC, Kotlovker D, Chung CY, Kim RY. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration: year 1 results of the focus study. Arch Ophthalmol. 2006;124:1532–1542. - PubMed
-
- Moja L, Lucenteforte E, Kwag KH, Bertele V, Campomori A, Chakravarthy U, D'Amico R, Dickersin K, Kodjikian L, Lindsley K, Loke Y, Maguire M, Martin DF, Mugelli A, Muhlbauer B, Puntmann I, Reeves B, Rogers C, Schmucker C, Subramanian ML, Virgili G. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014;9:CD011230. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

