Dutch Economic Value of Radium-223 in Metastatic Castration-Resistant Prostate Cancer
- PMID: 28866822
- PMCID: PMC5797195
- DOI: 10.1007/s40258-017-0350-x
Dutch Economic Value of Radium-223 in Metastatic Castration-Resistant Prostate Cancer
Erratum in
-
Correction to: Dutch Economic Value of Radium-223 in Metastatic Castration-Resistant Prostate Cancer.Appl Health Econ Health Policy. 2018 Feb;16(1):145. doi: 10.1007/s40258-017-0364-4. Appl Health Econ Health Policy. 2018. PMID: 29302922 Free PMC article.
Abstract
Background: The treatment of metastatic castration-resistant prostate cancer has changed with the introduction of radium-223, cabazitaxel, abiraterone and enzalutamide. To assess value for money, their cost effectiveness in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel from the Dutch societal perspective was investigated.
Methods: A cost-effectiveness analysis was conducted using efficacy, symptomatic skeletal-related event and safety data obtained from indirect treatment comparisons. Missing skeletal-related event data for cabazitaxel were conservatively assumed to be identical to radium-223. A Markov model combined these clinical inputs with Dutch-specific resource use and costs for metastatic castration-resistant prostate cancer treatment from a societal perspective. Total quality-adjusted life-years and costs in 2017 euros were calculated over a 5-year (lifetime) time horizon.
Results: Radium-223 resulted in €6092 and €4465 lower costs and 0.02 and 0.01 higher quality-adjusted life-years compared with abiraterone and cabazitaxel, respectively, demonstrating dominance of radium-223. Sensitivity analyses reveal a 64% (54%) chance of radium-223 being cost effective compared with abiraterone (cabazitaxel) at the informal €80,000 willingness-to-pay threshold. Compared with enzalutamide, radium-223 resulted in slightly lower quality-adjusted life-years (-0.06) and €7390 lower costs, revealing a 61% chance of radium-223 being cost effective compared with enzalutamide. The lower costs of radium-223 compared with abiraterone and enzalutamide are driven by lower drug costs and prevention of expensive skeletal-related events. Compared with cabazitaxel, the lower costs of radium-223 are driven by lower costs of the drug, administration and adverse events.
Conclusion: Radium-223 may be a less costly treatment strategy offering similar gains in health benefits compared with abiraterone, cabazitaxel and enzalutamide in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel from the Dutch societal perspective.
Conflict of interest statement
Funding
Mapi Group was financially supported by Bayer B. V. to perform the study.
Conflict of interest
M. L. Peters and J. G. Gaultney are Mapi Group employees and served as paid consultants to Bayer during the conduct of this study; A. Baka was a Mapi Group employee at the time of the study and served as a paid consultant to Bayer during the conduct of this study; C. de Meijer is a Bayer employee; D. Wyndaele was consulted by Bayer in advisory boards; W. Noordzij was consulted by Bayer in an advisory board; A. M. Leliveld-Kors was consulted by Bayer and Astellas in advisory boards; J. van den Bosch was consulted by Bayer in advisory boards; and H. P. van den Berg was consulted by Bayer in advisory boards.
Figures


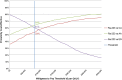
References
-
- Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–1159. doi: 10.1200/JCO.2007.12.4487. - DOI - PMC - PubMed
-
- Integraal Kankercentrum Nederland. Prostaatcarcinoom: Landelijke Richtlijn, Versie: 2.0.; 2014.
-
- Mottet N (Chair), Briers E (Patient Representative), Bolla M, Cornford P (Vice-chair), De Santis M, et al. Guideline prostate cancer. European Association of Urology. 2016. http://uroweb.org/guideline/prostate-cancer/. Accessed 25 Aug 2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

