Signaling and Gene Regulatory Networks in Mammalian Lens Development
- PMID: 28867048
- PMCID: PMC5627649
- DOI: 10.1016/j.tig.2017.08.001
Signaling and Gene Regulatory Networks in Mammalian Lens Development
Abstract
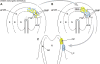
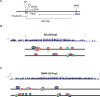
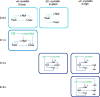
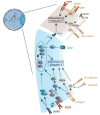
Ocular lens development represents an advantageous system in which to study regulatory mechanisms governing cell fate decisions, extracellular signaling, cell and tissue organization, and the underlying gene regulatory networks. Spatiotemporally regulated domains of BMP, FGF, and other signaling molecules in late gastrula-early neurula stage embryos generate the border region between the neural plate and non-neural ectoderm from which multiple cell types, including lens progenitor cells, emerge and undergo initial tissue formation. Extracellular signaling and DNA-binding transcription factors govern lens and optic cup morphogenesis. Pax6, c-Maf, Hsf4, Prox1, Sox1, and a few additional factors regulate the expression of the lens structural proteins, the crystallins. Extensive crosstalk between a diverse array of signaling pathways controls the complexity and order of lens morphogenetic processes and lens transparency.
Keywords: BMP; FGF; Pax6; Wnt signaling; cell determination; crystallins; differentiation; ectoderm; lens; pre-placodal region; retinoic acid.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

References
-
- Grainger RM. Embryonic lens induction: shedding light on vertebrate tissue determination. Trends Genet. 1992;8:349–55. - PubMed
-
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–53. - PubMed
-
- Beebe DC, Coats JM. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev. Biol. 2000;220:424–31. - PubMed
-
- Silla ZT, et al. Signals from the lens and Foxc1 regulate the expression of key genes during the onset of corneal endothelial development. Exp. Cell Res. 2014;322:381–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

