Spatial Clustering of de Novo Missense Mutations Identifies Candidate Neurodevelopmental Disorder-Associated Genes
- PMID: 28867141
- PMCID: PMC5591029
- DOI: 10.1016/j.ajhg.2017.08.004
Spatial Clustering of de Novo Missense Mutations Identifies Candidate Neurodevelopmental Disorder-Associated Genes
Abstract
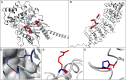
Haploinsufficiency (HI) is the best characterized mechanism through which dominant mutations exert their effect and cause disease. Non-haploinsufficiency (NHI) mechanisms, such as gain-of-function and dominant-negative mechanisms, are often characterized by the spatial clustering of mutations, thereby affecting only particular regions or base pairs of a gene. Variants leading to haploinsufficency might occasionally cluster as well, for example in critical domains, but such clustering is on the whole less pronounced with mutations often spread throughout the gene. Here we exploit this property and develop a method to specifically identify genes with significant spatial clustering patterns of de novo mutations in large cohorts. We apply our method to a dataset of 4,061 de novo missense mutations from published exome studies of trios with intellectual disability and developmental disorders (ID/DD) and successfully identify 15 genes with clustering mutations, including 12 genes for which mutations are known to cause neurodevelopmental disorders. For 11 out of these 12, NHI mutation mechanisms have been reported. Additionally, we identify three candidate ID/DD-associated genes of which two have an established role in neuronal processes. We further observe a higher intolerance to normal genetic variation of the identified genes compared to known genes for which mutations lead to HI. Finally, 3D modeling of these mutations on their protein structures shows that 81% of the observed mutations are unlikely to affect the overall structural integrity and that they therefore most likely act through a mechanism other than HI.
Copyright © 2017 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Gilissen C., Hehir-Kwa J.Y., Thung D.T., van de Vorst M., van Bon B.W., Willemsen M.H., Kwint M., Janssen I.M., Hoischen A., Schenck A. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–347. - PubMed
-
- Srour M., Caron V., Pearson T., Nielsen S.B., Lévesque S., Delrue M.A., Becker T.A., Hamdan F.F., Kibar Z., Sattler S.G. Gain-of-Function Mutations in RARB Cause Intellectual Disability with Progressive Motor Impairment. Hum. Mutat. 2016;37:786–793. - PubMed
-
- Hoischen A., van Bon B.W., Gilissen C., Arts P., van Lier B., Steehouwer M., de Vries P., de Reuver R., Wieskamp N., Mortier G. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat. Genet. 2010;42:483–485. - PubMed
-
- Schuurs-Hoeijmakers J.H., Oh E.C., Vissers L.E., Swinkels M.E., Gilissen C., Willemsen M.A., Holvoet M., Steehouwer M., Veltman J.A., de Vries B.B. Recurrent de novo mutations in PACS1 cause defective cranial-neural-crest migration and define a recognizable intellectual-disability syndrome. Am. J. Hum. Genet. 2012;91:1122–1127. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

