Calcified plaque modification alters local drug delivery in the treatment of peripheral atherosclerosis
- PMID: 28867375
- PMCID: PMC5955709
- DOI: 10.1016/j.jconrel.2017.08.037
Calcified plaque modification alters local drug delivery in the treatment of peripheral atherosclerosis
Abstract
Background: Calcific atherosclerosis is a major challenge to intraluminal drug delivery in peripheral artery disease (PAD).
Objectives: We evaluated the effects of orbital atherectomy on intraluminal paclitaxel delivery to human peripheral arteries with substantial calcified plaque.
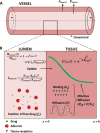
Methods: Diagnostic angiography and 3-D rotational imaging of five fresh human lower limbs revealed calcification in all main arteries. The proximal or distal segment of each artery was treated using an orbital atherectomy system (OAS) under simulated blood flow and fluoroscopy. Explanted arterial segments underwent either histomorphometric assessment of effect or tracking of 14C-labeled or fluorescent-labeled paclitaxel. Radiolabeled drug quantified bulk delivery and fluorescent label established penetration of drug over finer spatial domain in serial microscopic sections. Results were interpreted using a mathematical model of binding-diffusion mediated arterial drug distribution.
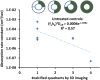
Results: Lesion composition affected paclitaxel absorption and distribution in cadaveric human peripheral arteries. Pretreatment imaging calcium scores in control femoropopliteal arterial segments correlated with a log-linear decline in the bulk absorption rate-constant of 14C-labeled, declining 5.5-fold per calcified quadrant (p=0.05, n=7). Compared to controls, OAS-treated femoropopliteal segments exhibited 180μm thinner intima (p<0.001), 45% less plaque calcification, and 2 log orders higher paclitaxel bulk absorption rate-constants. Correspondingly, fluorescent paclitaxel penetrated deeper in OAS-treated femoropopliteal segments compared to controls, due to a 70% increase in diffusivity (p<0.001).
Conclusions: These data illustrate that calcified plaque limited intravascular drug delivery, and controlled OAS treatment of calcific plaques resulted in greater drug permeability and improved adjunct drug delivery to diseased arteries.
Keywords: Atherectomy; Drug coated balloons; Drug eluting stents; Orbital atherectomy, calcified plaque; Paclitaxel; Peripheral artery disease.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation. 1992;86:64–70. - PubMed
-
- Adlakha S, Sheikh M, Wu J, Burket MW, Pandya U, Colyer W, Eltahawy E, Cooper CJ. Stent fracture in the coronary and peripheral arteries. J Interv Cardiol. 2010;23:411–419. - PubMed
-
- Micari A, Cioppa A, Vadala G, Castriota F, Liso A, Marchese A, Grattoni C, Pantaleo P, Cremonesi A, Rubino P, Biamino G. Clinical evaluation of a paclitaxel-eluting balloon for treatment of femoropopliteal arterial disease: 12-month results from a multicenter Italian registry. JACC Cardiovasc Interv. 2012;5:331–338. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

