From Original Antigenic Sin to the Universal Influenza Virus Vaccine
- PMID: 28867526
- PMCID: PMC5748348
- DOI: 10.1016/j.it.2017.08.003
From Original Antigenic Sin to the Universal Influenza Virus Vaccine
Abstract
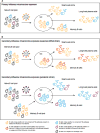
Antibody responses are essential for protection against influenza virus infection. Humans are exposed to a multitude of influenza viruses throughout their lifetime and it is clear that immune history influences the magnitude and quality of the antibody response. The 'original antigenic sin' concept refers to the impact of the first influenza virus variant encounter on lifelong immunity. Although this model has been challenged since its discovery, past exposure, and likely one's first exposure, clearly affects the epitopes targeted in subsequent responses. Understanding how previous exposure to influenza virus shapes antibody responses to vaccination and infection is critical, especially with the prospect of future pandemics and for the effective development of a universal influenza vaccine.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

