Achieving a Graded Immune Response: BTK Adopts a Range of Active/Inactive Conformations Dictated by Multiple Interdomain Contacts
- PMID: 28867612
- PMCID: PMC5629114
- DOI: 10.1016/j.str.2017.07.014
Achieving a Graded Immune Response: BTK Adopts a Range of Active/Inactive Conformations Dictated by Multiple Interdomain Contacts
Abstract
Capturing the functionally relevant forms of dynamic, multidomain proteins is extremely challenging. Bruton's tyrosine kinase (BTK), a kinase essential for B and mast cell function, has stubbornly resisted crystallization in its full-length form. Here, nuclear magnetic resonance and hydrogen-deuterium exchange mass spectrometry show that BTK adopts a closed conformation in dynamic equilibrium with open, active conformations. BTK lacks the phosphotyrosine regulatory tail of the SRC kinases, yet nevertheless achieves a phosphotyrosine-independent C-terminal latch. The unique proline-rich region is an internal "on" switch pushing the autoinhibited kinase toward its active state. Newly identified autoinhibitory contacts in the BTK pleckstrin homology domain are sensitive to phospholipid binding, which induces large-scale allosteric changes. The multiplicity of these regulatory contacts suggests a clear mechanism for gradual or "analog" kinase activation as opposed to a binary "on/off" switch. The findings illustrate how previously modeled information for recalcitrant full-length proteins can be expanded and validated with a convergent multidisciplinary experimental approach.
Keywords: BTK; TEC; autoinhibition; conformational equilibria; crystallization-resistant proteins; kinase regulation.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
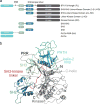

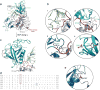
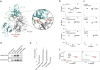




Comment in
-
Switching on BTK-One Domain at a Time.Structure. 2017 Oct 3;25(10):1469-1470. doi: 10.1016/j.str.2017.09.012. Epub 2017 Oct 3. Structure. 2017. PMID: 28978404
References
-
- Andreotti AH, Bunnell SC, Feng S, Berg LJ, Schreiber SL. Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature. 1997;385:93–97. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

