FVIIa-sTF and Thrombin Inhibitory Activities of Compounds Isolated from Microcystis aeruginosa K-139
- PMID: 28867804
- PMCID: PMC5618414
- DOI: 10.3390/md15090275
FVIIa-sTF and Thrombin Inhibitory Activities of Compounds Isolated from Microcystis aeruginosa K-139
Abstract
The rise of bleeding and bleeding complications caused by oral anticoagulant use are serious problems nowadays. Strategies that block the initiation step in blood coagulation involving activated factor VII-tissue factor (fVIIa-TF) have been considered. This study explores toxic Microcystis aeruginosa K-139, from Lake Kasumigaura, Ibaraki, Japan, as a promising cyanobacterium for isolation of fVIIa-sTF inhibitors. M. aeruginosa K-139 underwent reversed-phase solid-phase extraction (ODS-SPE) from 20% MeOH to MeOH elution with 40%-MeOH increments, which afforded aeruginosin K-139 in the 60% MeOH fraction; micropeptin K-139 and microviridin B in the MeOH fraction. Aeruginosin K-139 displayed an fVIIa-sTF inhibitory activity of ~166 µM, within a 95% confidence interval. Micropeptin K-139 inhibited fVIIa-sTF with EC50 10.62 µM, which was more efficient than thrombin inhibition of EC50 26.94 µM. The thrombin/fVIIa-sTF ratio of 2.54 in micropeptin K-139 is higher than those in 4-amidinophenylmethane sulfonyl fluoride (APMSF) and leupeptin, when used as positive controls. This study proves that M. aeruginosa K-139 is a new source of fVIIa-sTF inhibitors. It also opens a new avenue for micropeptin K-139 and related depsipeptides as fVIIa-sTF inhibitors.
Keywords: LC-MS; Microcystis; aeruginosin K-139; blood coagulation cascade; fVIIa-sTF inhibitors; micropeptin K-139; microviridin B; thrombin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

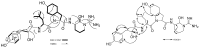




References
-
- Palareti G., Leali N., Coccheri S., Poggi M., Manotti C., D’Angelo A., Pengo V., Erba N., Moia M., Ciavarella N., et al. Bleeding complications of oral anticoagulant treatment: An inception-cohort, prospective collaborative study (ISCOAT) Lancet. 1996;348:423–428. doi: 10.1016/S0140-6736(96)01109-9. - DOI - PubMed
-
- Anas A.R.J., Harada K.-I. Evaluation of Serine Protease Inhibitors as Potent FVIIa-sTF Inhibitors in the Blood Coagulation Cascade. Lett. Drug Des. Discov. 2016;13:3–23. doi: 10.2174/1570180812666150608230214. - DOI
-
- Nakagura T., Tabata K., Kira K., Hirota S., Clark R., Matsuura F., Hiyoshi H. Selective tissue factor/factor VIIa Inhibitor, ER-410660, and its prodrug, E5539, have anti-venous and anti-arterial thrombotic effects with a low risk of bleeding. Thromb. Res. 2013;132:271–279. doi: 10.1016/j.thromres.2013.06.012. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

