Synthesis of Glycosides by Glycosynthases
- PMID: 28867807
- PMCID: PMC6151457
- DOI: 10.3390/molecules22091434
Synthesis of Glycosides by Glycosynthases
Abstract
The many advances in glycoscience have more and more brought to light the crucial role of glycosides and glycoconjugates in biological processes. Their major influence on the functionality and stability of peptides, cell recognition, health and immunity and many other processes throughout biology has increased the demand for simple synthetic methods allowing the defined syntheses of target glycosides. Additional interest in glycoside synthesis has arisen with the prospect of producing sustainable materials from these abundant polymers. Enzymatic synthesis has proven itself to be a promising alternative to the laborious chemical synthesis of glycosides by avoiding the necessity of numerous protecting group strategies. Among the biocatalytic strategies, glycosynthases, genetically engineered glycosidases void of hydrolytic activity, have gained much interest in recent years, enabling not only the selective synthesis of small glycosides and glycoconjugates, but also the production of highly functionalized polysaccharides. This review provides a detailed overview over the glycosylation possibilities of the variety of glycosynthases produced until now, focusing on the transfer of the most common glucosyl-, galactosyl-, xylosyl-, mannosyl-, fucosyl-residues and of whole glycan blocks by the different glycosynthase enzyme variants.
Keywords: biocatalysis; glycosidase; glycoside; glycosylation; glycosynthase; polysaccharide; synthesis.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures

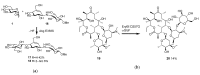

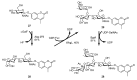

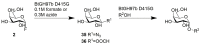
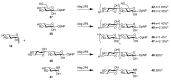
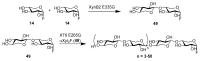
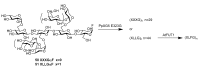
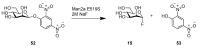



References
-
- Stevens J., Blixt O., Glaser L., Taubenberger J.K., Palese P., Paulson J.C., Wilson I.A. Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J. Mol. Biol. 2006;355:1143–1155. doi: 10.1016/j.jmb.2005.11.002. - DOI - PubMed
-
- Douglas N.L., Ley S.V., Lucking U., Warriner S.L. Tuning glycoside reactivity: New tool for efficient oligosaccharide synthesis. J. Chem. Soc. Perkin Trans. 1. 1998:51–66. doi: 10.1039/a705275h. - DOI
-
- Grice P., Ley S.V., Pietruszka J., Priepke H.W.M., Walther E.P.E. Tuning the reactivity of glycosides: Efficient one-pot oligosaccharide synthesis1. Synlett. 1995;1995:781–784. doi: 10.1055/s-1995-5052. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

