Distinct Seasonal Patterns of Bacterioplankton Abundance and Dominance of Phyla α- Proteobacteria and Cyanobacteria in Qinhuangdao Coastal Waters Off the Bohai Sea
- PMID: 28868051
- PMCID: PMC5563310
- DOI: 10.3389/fmicb.2017.01579
Distinct Seasonal Patterns of Bacterioplankton Abundance and Dominance of Phyla α- Proteobacteria and Cyanobacteria in Qinhuangdao Coastal Waters Off the Bohai Sea
Abstract
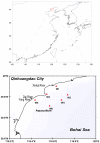
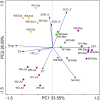
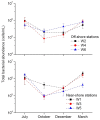
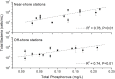
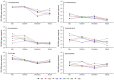
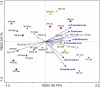
Qinhuangdao coastal waters in northern China are heavily impacted by anthropogenic and natural activities, and we anticipate a direct influence of the impact on the bacterioplankton abundance and diversity inhabiting the adjacent coastal areas. To ascertain the anthropogenic influences, we first evaluated the seasonal abundance patterns and diversity of bacterioplankton in the coastal areas with varied levels of natural and anthropogenic activities and then analyzed the environmental factors which influenced the abundance patterns. Results indicated distinct patterns in bacterioplankton abundance across the warm and cold seasons in all stations. Total bacterial abundance in the stations ranged from 8.67 × 104 to 2.08 × 106 cells/mL and had significant (p < 0.01) positive correlation with total phosphorus (TP), which indicated TP as the key monitoring parameter for anthropogenic impact on nutrients cycling. Proteobacteria and Cyanobacteria were the most abundant phyla in the Qinhuangdao coastal waters. Redundancy analysis revealed significant (p < 0.01) influence of temperature, dissolved oxygen and chlorophyll a on the spatiotemporal abundance pattern of α-Proteobacteria and Cyanobacteria groups. Among the 19 identified bacterioplankton subgroups, α-Proteobacteria (phylum Proteobacteria) was the dominant one followed by Family II (phylum Cyanobacteria), representing 19.1-55.2% and 2.3-54.2% of total sequences, respectively. An inverse relationship (r = -0.82) was observed between the two dominant subgroups, α-Proteobacteria and Family II. A wide range of inverse Simpson index (10.2 to 105) revealed spatial heterogeneity of bacterioplankton diversity likely resulting from the varied anthropogenic and natural influences. Overall, our results suggested that seasonal variations impose substantial influence on shaping bacterioplankton abundance patterns. In addition, the predominance of only a few cosmopolitan species in the Qinhuangdao coastal wasters was probably an indication of their competitive advantage over other bacterioplankton groups in the degradation of anthropogenic inputs. The results provided an evidence of their ecological significance in coastal waters impacted by seasonal inputs of the natural and anthropogenic matter. In conclusion, the findings anticipate future development of effective indicators of coastal health monitoring and subsequent management strategies to control the anthropogenic inputs in the Qinhuangdao coastal waters.
Keywords: anthropogenic impacts; bacterioplankton abundance; environmental variables; phylogenetic diversity; redundancy analysis; seasonal variations.
Figures

Similar articles
-
Seasonal and anthropogenic influences on bacterioplankton communities: ecological impacts in the coastal waters of Qinhuangdao, Northern China.Front Microbiol. 2024 Jun 19;15:1431548. doi: 10.3389/fmicb.2024.1431548. eCollection 2024. Front Microbiol. 2024. PMID: 38962120 Free PMC article.
-
Seasonal and spatial dynamics of bacterioplankton communities in a brackish water coastal lagoon.Sci Total Environ. 2020 Feb 25;705:134729. doi: 10.1016/j.scitotenv.2019.134729. Epub 2019 Dec 5. Sci Total Environ. 2020. PMID: 31838414
-
[Effects of Organic Pollutants on the Bacterioplankton Community in Hangzhou Bay].Huan Jing Ke Xue. 2018 Aug 8;39(8):3640-3648. doi: 10.13227/j.hjkx.201712186. Huan Jing Ke Xue. 2018. PMID: 29998670 Chinese.
-
Diversity of rare and abundant bacteria in surface waters of the Southern Adriatic Sea.Mar Genomics. 2014 Oct;17:9-15. doi: 10.1016/j.margen.2014.04.002. Epub 2014 Apr 13. Mar Genomics. 2014. PMID: 24736045
-
Seasonal influence of scallop culture on nutrient flux, bacterial pathogens and bacterioplankton diversity across estuaries off the Bohai Sea Coast of Northern China.Mar Pollut Bull. 2017 Nov 15;124(1):411-420. doi: 10.1016/j.marpolbul.2017.07.062. Epub 2017 Aug 2. Mar Pollut Bull. 2017. PMID: 28779889
Cited by
-
Inter-phylum negative interactions affect soil bacterial community dynamics and functions during soybean development under long-term nitrogen fertilization.Stress Biol. 2021 Nov 26;1(1):15. doi: 10.1007/s44154-021-00015-0. Stress Biol. 2021. PMID: 37676329 Free PMC article.
-
Phylogenetic diversity and functional potential of large and cell-associated viruses in the Bay of Bengal.mSphere. 2023 Dec 20;8(6):e0040723. doi: 10.1128/msphere.00407-23. Epub 2023 Oct 30. mSphere. 2023. PMID: 37902318 Free PMC article.
-
Bacterioplankton community variation in Bohai Bay (China) is explained by joint effects of environmental and spatial factors.Microbiologyopen. 2020 Apr;9(4):e997. doi: 10.1002/mbo3.997. Epub 2020 Feb 5. Microbiologyopen. 2020. PMID: 32022464 Free PMC article.
-
Bacterial taxonomic and functional profiles from Bohai Sea to northern Yellow Sea.Front Microbiol. 2023 Feb 23;14:1139950. doi: 10.3389/fmicb.2023.1139950. eCollection 2023. Front Microbiol. 2023. PMID: 36910186 Free PMC article.
-
Unveiling the Microbiome Landscape: A Metagenomic Study of Bacterial Diversity, Antibiotic Resistance, and Virulence Factors in the Sediments of the River Ganga, India.Antibiotics (Basel). 2023 Dec 14;12(12):1735. doi: 10.3390/antibiotics12121735. Antibiotics (Basel). 2023. PMID: 38136769 Free PMC article.
References
-
- Al-Rifaie K., Al-Yamani F., Polikarpov I. (2008). First study of the bacterioplankton distribution in the Northwestern Arabian Gulf. Mar. Ecol. J. 574 43–48.
-
- Barrera-Alba J. J., Gianesella S. M. F., Moser G. A. O., Saldanha-Corrêa F. M. P. (2009). Influence of allochthonous organic matter on bacterioplankton biomass and activity in a eutrophic, sub-tropical estuary. Estuar. Coast. Shelf Sci. 82 84–94. 10.1016/j.ecss.2008.12.020 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

