Amniocentesis and chorionic villus sampling for prenatal diagnosis
- PMID: 28869276
- PMCID: PMC6483702
- DOI: 10.1002/14651858.CD003252.pub2
Amniocentesis and chorionic villus sampling for prenatal diagnosis
Abstract
Background: During pregnancy, fetal cells suitable for genetic testing can be obtained from amniotic fluid by amniocentesis (AC), placental tissue by chorionic villus sampling (CVS), or fetal blood. A major disadvantage of second trimester amniocentesis is that the results are available relatively late in pregnancy (after 16 weeks' gestation). Earlier alternatives are chorionic villus sampling (CVS) and early amniocentesis, which can be performed in the first trimester of pregnancy.
Objectives: The objective of this review was to compare the safety and accuracy of all types of AC (i.e. early and late) and CVS (e.g. transabdominal, transcervical) for prenatal diagnosis.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (3 March 2017), ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP; 3 March 2017), and reference lists of retrieved studies.
Selection criteria: All randomised trials comparing AC and CVS by either transabdominal or transcervical route.
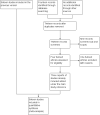
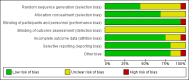
Data collection and analysis: Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. The quality of the evidence was assessed using the GRADE approach.
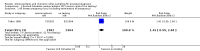
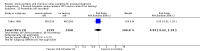
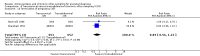
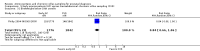
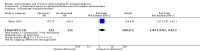
Main results: We included a total of 16 randomised studies, with a total of 33,555 women, 14 of which were deemed to be at low risk of bias. The number of women included in the trials ranged from 223 to 4606.Studies were categorized into six comparisons: 1. second trimester AC versus control; 2. early versus second trimester AC; 3. CVS versus second trimester AC; 4. CVS methods; 5. Early AC versus CVS; and 6. AC with or without ultrasound.One study compared second trimester AC with no AC (control) in a low risk population (women = 4606). Background pregnancy loss was around 2%. Second trimester AC compared to no testing increased total pregnancy loss by another 1%. The confidence intervals (CI) around this excess risk were relatively large (3.2% versus 2.3 %, average risk ratio (RR) 1.41, 95% CI 0.99 to 2.00; moderate-quality evidence). In the same study, spontaneous miscarriages were also higher (2.1% versus 1.3%; average RR 1.60, 95% CI 1.02 to 2.52; high-quality evidence). The number of congenital anomalies was similar in both groups (2.0% versus 2.2%, average RR 0.93, 95% CI 0.62 to 1.39; moderate-quality evidence).One study (women = 4334) found that early amniocentesis was not a safe early alternative compared to second trimester amniocentesis because of increased total pregnancy losses (7.6% versus 5.9%; average RR 1.29, 95% CI 1.03 to 1.61; high-quality evidence), spontaneous miscarriages (3.6% versus 2.5%, average RR 1.41, 95% CI 1.00 to 1.98; moderate-quality evidence), and a higher incidence of congential anomalies, including talipes (4.7% versus 2.7%; average RR 1.73, 95% CI 1.26 to 2.38; high-quality evidence).When pregnancy loss after CVS was compared with second trimester AC, there was a clinically significant heterogeneity in the size and direction of the effect depending on the technique used (transabdominal or transcervical), therefore, the results were not pooled. Only one study compared transabdominal CVS with second trimester AC (women = 2234). They found no clear difference between the two procedures in the total pregnancy loss (6.3% versus 7%; average RR 0.90, 95% CI 0.66 to 1.23, low-quality evidence), spontaneous miscarriages (3.0% versus 3.9%; average RR 0.77, 95% CI 0.49 to 1.21; low-quality evidence), and perinatal deaths (0.7% versus 0.6%; average RR 1.18, 95% CI 0.40 to 3.51; low-quality evidence). Transcervical CVS may carry a higher risk of pregnancy loss (14.5% versus 11.5%; average RR 1.40, 95% CI 1.09 to 1.81), but the results were quite heterogeneous.Five studies compared transabdominal and transcervical CVS (women = 7978). There were no clear differences between the two methods in pregnancy losses (average RR 1.16, 95% CI 0.81 to 1.65; very low-quality evidence), spontaneous miscarriages (average RR 1.68, 95% CI 0.79 to 3.58; very low-quality evidence), or anomalies (average RR 0.68, 95% CI 0.41 to 1.12; low-quality evidence). We downgraded the quality of the evidence to low due to heterogeneity between studies. Transcervical CVS may be more technically demanding than transabdominal CVS, with more failures to obtain sample (2.0% versus 1.1%; average RR 1.79, 95% CI 1.13 to 2.82, moderate-quality evidence).Overall, we found low-quality evidence for outcomes when early amniocentesis was compared to transabdominal CVS. Spontaneous miscarriage was the only outcome supported by moderate-quality evidence, resulting in more miscarriages after early AC compared with transabdominal CVS (2.3% versus 1.3%; average RR 1.73, 95% CI 1.15 to 2.60). There were no clear differences in pregnancy losses (average RR 1.15, 95% CI 0.86 to 1.54; low-quality evidence), or anomalies (average RR 1.14, 95% CI 0.57 to 2.30; very low-quality evidence).We found one study that examined AC with or without ultrasound, which evaluated a type of ultrasound-assisted procedure that is now considered obsolete.
Authors' conclusions: Second trimester amniocentesis increased the risk of pregnancy loss, but it was not possible to quantify this increase precisely from only one study, carried out more than 30 years ago.Early amniocentesis was not as safe as second trimester amniocentesis, illustrated by increased pregnancy loss and congenital anomalies (talipes). Transcervical chorionic villus sampling compared with second trimester amniocentesis may be associated with a higher risk of pregnancy loss, but results were quite heterogeneous.Diagnostic accuracy of different methods could not be assessed adequately because of incomplete karyotype data in most studies.
Conflict of interest statement
Zarko Alfirevic: Zarko Alfirevic is Director of the Harris Wellbeing Preterm Birth Centre, which is grant funded by the charity Wellbeing of Women. This grant is administered by the University of Liverpool, and Zarko Alfirevic is not paid directly. He is the principal investigator or co‐investigator on several grants from public funders, including National Institute of Health Research, British Medical Association, European Commission, and WHO. He has received research support in the past from Perkin Elmer and Alere for research related to pre‐eclampsia and preterm birth prevention. These grants were administered by his employers and ZA did not benefit directly. ZA is also a Co‐coordinating Editor of Cochrane Pregnancy and Childbirth.
Kate Navaratnam: none known.
Faris Mujezinovic: none known.
Figures



























































































































Update of
-
Amniocentesis and chorionic villus sampling for prenatal diagnosis.Cochrane Database Syst Rev. 2003;(3):CD003252. doi: 10.1002/14651858.CD003252. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2017 Sep 04;9:CD003252. doi: 10.1002/14651858.CD003252.pub2. PMID: 12917956 Free PMC article. Updated.
References
References to studies included in this review
Ammala 1993 (MRC Finland) {unpublished data only}
-
- Ammala P, Hiilesmaa V, Teramo K, Koskul HV. Randomised trial comparing first trimester transcervical chorion villus sampling and second trimester amniocentesis. 13th World Congress of Gynaecology and Obstetrics (FIGO); 1991 Sept 15‐20; Singapore. 1991:22.
-
- Ammala P, Hiilesmaa VK, Liukkonen S, Saisto T, Teramo K, Koskull H. Randomized trial comparing first‐trimester transcervical chorionic villus sampling and second‐trimester amniocentesis. Prenatal Diagnosis 1993;13:919‐27. - PubMed
Borrell 1999 {published data only}
-
- Borrell A, Fortuny A, Lazaro A, Costa D, Seres A, Pappa S, et al. First‐trimester transcervical chorionic villus sampling by biopsy forceps versus mid‐trimester amniocentesis: a randomized controlled trial project. Prenatal Diagnosis 1999;19:1138‐42. - PubMed
-
- Borrell A, Fortuny A, Lazaro I, Seres A, Papa S, Costa D, et al. Trancervical chorial biopsy versus amniocentesis: evaluation of fetal loss in a randomized study [Biopsia corial transcervical versus amniocentesis: evaluación de la pérdida fetal en un estudio randomizado]. Progresos de Obstetricia y Ginecología 2000;43(4):169‐75.
Bovicelli 1986 {published data only}
-
- Bovicelli L, Rizzo N, Montacuti V, Morandi R. Transabdominal vs transcervical routes for chorionic villus sampling. Lancet 1986;2:290. - PubMed
Brambati 1991 {published data only}
-
- Brambati B. Advantages and risks of transcervical and transabdominal CVS methods. 11th European Congress of Perinatal Medicine; 1988 April 10‐13; Rome, Italy. 1988:141.
-
- Brambati B, Oldrini A, Lanzani A, Terzian E, Tognoni G. Transabdominal vs transcervical chorionic villus sampling: a randomized trial. Human Reproduction 1988;3:811‐3. [abstract] - PubMed
-
- Brambati B, Terzian E, Tognoni G. Randomized clinical trial of transabdominal vs transcervical chorionic villus sampling methods. Prenatal Diagnosis 1991;11:285‐93. - PubMed
Canada 1989 {published data only}
-
- Canadian Collaborative CVS‐Amniocentesis Clinical Trial Group. Canadian multi‐centre randomized clinical trial of chorion villus sampling and amniocentesis: first report. Lancet 1989;1:1‐6. - PubMed
-
- Feeny D, Townsend M, Furlong W, Tomkins DJ, Robinson GE, Torrance GW, et al. Health‐related quality‐of‐life assessment of prenatal diagnosis: chorionic villi sampling and amniocentesis. Genetic Testing 2002;6:39‐46. - PubMed
-
- Hamerton JL. Chorionic villus sampling vs amniocentesis. Lancet 1989;1:678. - PubMed
-
- Kaplan P, Normandin JJ, Wilson GN, Plauchu H, Lippman A, Vekemans M. Malformations and minor anomalies in children whose mothers had prenatal diagnosis: comparison between CVS and amniocentesis. American Journal of Medical Genetics 1990;37(3):366‐70. [MEDLINE: ] - PubMed
-
- Lippman A, Tomkins DJ, Shima J, Hamerton JL. Canadian multicentre randomized clinical trial of chorion villus sampling and amniocentesis. Prenatal Diagnosis 1992;12:385‐408. - PubMed
CEMAT 1998 {published data only}
-
- Johnson J, Wilson R. CEMAT (Canadian early (EA) vs. midtrimester (MA) amniocentesis trial) prospective randomized evaluation: amniocentesis procedure details. American Journal of Obstetrics and Gynecology 1998;178(1):22. [abstract]
-
- Johnson JM, Wilson RD, Singer J, Winsor E, Harman C, Armson BA, et al. Technical factors in early amniocentesis predict adverse outcome. Results of the Canadian early (ea) versus mid‐trimester (ma) amniocentesis trial. Prenatal Diagnosis 1999;19:732‐8. - PubMed
-
- Johnson JM, Wilson RD, Winsor E, Kalousek D, Soanes S, Sorensen S, et al. The early amniocentesis study: a randomized clinical trial of early amniocentesis vs mid‐trimester amniocentesis. International Journal of Gynaecology and Obstetrics 1994;46:41. [abstract]
-
- Johnson JM, Wilson RD, Winsor EJ, Singer J, Dansereau J, Kalousek DK. The early amniocentesis study: a randomized clinical trial of early amniocentesis versus midtrimester amniocentesis. Fetal Diagnosis and Therapy 1996;11(2):85‐93. - PubMed
Jackson 1992 {published data only}
-
- Collaborative. Transcervical and transabdominal chorionic villus sampling are comparably safe procedures for first trimester prenatal diagnosis: preliminary analysis. American Journal of Human Genetics 1990;47:A278.
-
- Jackson L, Zachary J, Fowler S, Desnick R, Golbus M, Ledbetter D, et al. A randomized comparison of transcervical and transabdominal chorionic‐villus sampling. New England Journal of Medicine 1992;327:594‐8. - PubMed
Leiden 1998 {published data only}
-
- Nagel HTC, Vandenbussche FPHA, Keirse MJNC, Oepkes D, Oosterwijk JC, Beverstock G, et al. Amniocentesis before 14 completed weeks as an alternative to transabdominal chorionic villus sampling: a controlled trial with infant follow‐up. Prenatal Diagnosis 1998;18(5):465‐75. - PubMed
-
- Vandenbussche F, Kanhai H, Keirse M. Safety of early amniocentesis. Lancet 1994;344(8):1032. - PubMed
MRC 1991 {published data only}
-
- MRC working party on the evaluation of chorion villus sampling. Medical Research Council European trial of chorion villus sampling. Lancet 1991;337:1491‐9. - PubMed
Nicolaides 1994 (King's) {published data only}
-
- Byrne D, Marks K, Azar G, Nicolaides K. Randomized study of early amniocentesis vs chorionic villus sampling: a technical and cytogenetic comparison of 650 patients. Ultrasound in Obstetrics & Gynecology 1991;1:235‐40. - PubMed
-
- Greenough A, Yuksel B, Naik S, Cheeseman P, Nicolaides KH. Invasive antenatal procedures and requirement for neonatal intensive care unit admission. European Journal of Pediatrics 1997;156(7):550‐2. - PubMed
-
- Nicolaides K, Lourdes Brizot M, Patel F, Snijders R. Comparison of chorionic villus sampling and amniocentesis for fetal karyotyping at 10‐13 weeks' gestation. Lancet 1994;344:435‐9. - PubMed
-
- Nicolaides KH, Brizot ML, Patel F, Snijders R. Comparison of chorion villus sampling and early amniocentesis for karyotyping in 1,492 singleton pregnancies. Fetal Diagnosis and Therapy 1996;11(1):9‐15. - PubMed
-
- Thilaganathan B, Snijders R, Nicolaides K. Randomised study of chorionic villus sampling vs amniocentesis at 10‐13 weeks gestation. International Journal of Gynecology & Obstetrics 1994;46:75.
Nolan 1981 {published data only}
-
- Nolan GH, Schmickel RD, Chantaratherakitti P, Knickerbocker C, Hamman J, Louwsma G. The effect of ultrasonography on midtrimester genetic amniocentesis complications. American Journal of Obstetrics and Gynecology 1981;140:531‐4. - PubMed
Philip 2004 (NICHD EATA) {published data only}
-
- NCT00065897. Randomized trial of 11‐14 week amniocentesis and transabdominal chorionic villus sampling (TA CVS). clinicaltrials.gov/ct2/show/NCT00065897 (first received 1 August 2003).
-
- Philip J, Silver RK, Wilson RD, Thom EA, Zachary JM, Mohide P, et al. Late first‐trimester invasive prenatal diagnosis‐‐secondary publication. An international randomized trial [Invasiv prøvetagning ved prænatal diagnostik sent i første trimester ‐ sekundærpublikation]. Ugeskrift for Laeger 2005;167(11):1293‐6. [MEDLINE: ] - PubMed
-
- Philip J, Silver RK, Wilson RD, Thom EA, Zachary JM, Mohide P, et al. Late first‐trimester invasive prenatal diagnosis: results of an international randomized trial. Obstetrics and Gynecology 2004;103(6):1164‐73. [MEDLINE: ] - PubMed
-
- Philip J, NICHD EATA Study Group. Greater risk associated with early amniocentesis compared to chorionic villus sampling: an international randomized trial. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S69. [abstract]
Smidt‐Jensen 1993 (Denmark) {published data only}
-
- Smidt‐Jensen S. Randomised comparison of amniocentesis and transabdominal and transcervical chorionic villus sampling. Geburtshilfe und Frauenheilkunde 1993;53:822. - PubMed
-
- Smidt‐Jensen S, Permin M, Philip J. Sampling success and risk by transabdominal chorionic villus sampling, transcervical chorionic villus sampling and amniocentesis: a randomized study. Ultrasound in Obstetrics & Gynecology 1991;1:86‐90. - PubMed
-
- Smidt‐Jensen S, Permin M, Philip J, Lundsteen C, Zachary J, Fowler S, et al. Randomized comparison of amniocentesis and transabdominal and transcervical chorionic villus sampling. Lancet 1992;340:1237‐44. - PubMed
-
- Smidt‐Jensen S, Philip J. Comparison of transabdominal and transcervical CVS and amniocentesis: sampling success and risk. Prenatal Diagnosis 1991;11:529‐37. - PubMed
-
- Smidt‐Jensen SL, Permin M, Philip J, Lundsteen C, Gruning K, Zachary J, et al. Amniocentesis, transabdominal and transcervical chorionic villus sampling compared in a randomised trial. Ugeskrift for Laeger 1993;155:1446‐56. - PubMed
Sundberg 1997 (Copenhagen) {published data only}
-
- Sundberg K. Potential and problems with ultrasound in fetal diagnosis at 10‐11 weeks and amniocentesis at 12 weeks. Acta Obstetricia et Gynecologica Scandinavica Supplement 1994;73(161):SP31.
-
- Sundberg K, Bang J, Smidt Jensen S, Brocks V, Lundsteen C, Parner J, et al. Randomised study of risk of fetal loss related to early amniocentesis versus chorionic villus sampling. Lancet 1997;350(9079):697‐703. - PubMed
-
- Sundberg K, Lundsteen C, Philip J. Comparison of cell cultures, chromosome quality and karyotype obtained after CVS and early amniocentesis with filter technique. Prenatal Diagnosis 1999;19:12‐6. - PubMed
Tabor 1986 {published data only}
-
- Tabor A, Bang J, Norgaard‐Pedersen B. Feto‐maternal haemorrhage associated with genetic amniocentesis: results of a randomized trial. British Journal of Obstetrics and Gynaecology 1987;94:528‐34. - PubMed
-
- Tabor A, Madsen M, Obel EB, Philip J, Bang J, Norgaard‐Pedersen B. Randomised controlled trial of genetic amniocentesis in 4606 low‐risk women. Lancet 1986;1:1287‐93. - PubMed
-
- Tabor A, Philip J. Incidence of fetal chromosome abnormalities in 2264 low‐risk women. Prenatal Diagnosis 1987;7(5):355‐62. [MEDLINE: ] - PubMed
Tomassini 1988 {published data only}
-
- Tomassini A, Campagna G, Paolucci M, Ferrario D, Zarini E, Tibiletti MG, et al. Transvaginal CVS vs transabdominal CVS (our randomised cases). XI European Congress of Perinatal Medicine; 1988 April 10‐13; Rome, Italy. 1988:1101‐4.
References to studies excluded from this review
Cederholm 1997 (Uppsala) {published data only}
-
- Cederholm M, Axelsson O. A prospective comparative study on transabdominal chorionic villus sampling and amniocentesis performed at 10‐13 weeks' gestation. Prenatal Diagnosis 1997;17(4):311‐7. - PubMed
Chang 1994 {published data only}
-
- Chang JC. Simultaneous mid‐trimester AC & placental biopsy ‐ experience of 92 cases. International Journal of Gynaecology and Obstetrics 1994;46:41.
Corrado 2002 {published data only}
-
- Corrado F, Dugo C, Cannata ML, Bartolo M, Scilipoti A, Stella NC. A randomised trial of progesterone prophylaxis after midtrimester amniocentesis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2002;100(2):196‐8. [MEDLINE: ] - PubMed
Fischer 2000 {published data only}
-
- Fischer R, Bianculli K, Sehdev H, Hediger M. Does light pressure effleurage reduce pain and anxiety associated with genetic amniocentesis: a randomized clinical trial.. American Journal of Obstetrics and Gynecology 2000;182(1):S186. - PubMed
-
- Fisher RL, Bianculli KW, Sehdev H, Hediger ML. Does light pressure effleurage reduce pain and anxiety associated with genetic amniocentesis? A randomized clinical trial. Journal of Maternal‐fetal Medicine 2000;9:294‐7. - PubMed
Gordon 2007 {published data only}
-
- Gordon MC, Ventura‐Braswell A, Higby K, Ward JA. Does local anesthesia decrease pain perception in women undergoing amniocentesis?. American Journal of Obstetrics and Gynecology 2007;196(1):55.e1‐4. [MEDLINE: ] - PubMed
Hewison 2006 (ARIA Trial) {published data only}
-
- Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al. Amniocentesis results: investigation of anxiety. The ARIA trial. Health Technology Assessment 2006;10(50):1‐266. [MEDLINE: ] - PubMed
-
- Hewison J, Nixon J, Fountain J, Hawkins K, Jones CR, Mason G, et al. A randomised trial of two methods of issuing prenatal test results: the ARIA (amniocentesis results: investigation of anxiety) trial. BJOG: an international journal of obstetrics and gynaecology 2007;114(4):462‐8. [MEDLINE: ] - PubMed
Horovitz 1994 {published data only}
-
- Horovitz J, Verdier G, Roux D, Hocke C, Taine L, Maugey B, et al. Prenatal diagnosis in multiple pregnancies: transabdominal CVS (59 cases) vs amniocentesis (56 cases). International Journal of Gynaecology and Obstetrics 1994;46:41.
ISRCTN18010960 {published data only}
-
- ISRCTN18010960. Amniocentesis before rescue cerclage. isrctn.com/ISRCTN18010960 (first received 30 October 2007).
Ketupanya 1997 {published data only}
-
- Ketupanya A, Mutamara S, Vuthivong J, Kungvanpong D, Samativatr S. Amnifiltration in early amniocentesis for cytogenetic evaluation: randomized clinical trial. Acta Obstetricia et Gynecologica Scandinavica 1997;76(167):40.
Leach 1978 {published data only}
-
- Leach G, Chang A, Morrison J. A controlled trial of puncture sites for amniocentesis. British Journal of Obstetrics and Gynaecology 1978;85:328‐31. - PubMed
Leung 2002 {published data only}
-
- Leung WC, Lam YH, Wong Y, Lau ET, Tang MH. The effect of fast reporting by amnio‐PCR on anxiety levels in women with positive biochemical screening for Down syndrome − a randomized controlled trial. Prenatal Diagnosis 2002;22(3):256‐9. [MEDLINE: ] - PubMed
Levine 1977 {published data only}
-
- Levine SC, Filly RA, Golbus MS. Ultrasonography for guidance of amniocentesis in genetic counselling. Clinical Genetics 1978;14:133‐8. - PubMed
Pistorius 1998 {published data only}
-
- Pistorius L, Howarth G, Freislich L, Pattison R, Mantel G, Honey E, et al. Amniocentesis and the taptest in proteinuric hypertension in pregnancy "tappet"; a randomised controlled trial. 17th Conference on Priorities in Perinatal Care; 1998 March 5; South Africa. 1998:96.
Shalev 1994 {published data only}
-
- Shalev E, Weiner E, Yanai N, Shneur Y, Cohen H. Comparison of first‐trimester transvaginal amniocentesis with chorionic villus sampling and mid‐trimester amniocentesis. Prenatal Diagnosis 1994;14(4):279‐83. [MEDLINE: ] - PubMed
Shulman 1990 {published data only}
-
- Shulman LP, Meyers CM, Simpson JL, Andersen RN, Tolley EA, Elias S. Fetomaternal transfusion depends on amount of chorionic villi aspirated but not on method of chorionic villus sampling. American Journal of Obstetrics and Gynecology 1990;162:1185‐8. - PubMed
SIlver 2005 {published data only}
-
- Silver RK, Wilson RD, Philip J. Transplacental prenatal diagnosis at 13‐14 weeks may increase the risk of gestational hypertension/preeclampsia. American Journal of Obstetrics and Gynecology 2003;189(6):S87.
-
- Silver RK, Wilson RD, Philip J, Thom EA, Zachary JM, Mohide P. Late first‐trimester placental disruption and subsequent gestational hypertension/preeclampsia. Obstetrics and Gynecology 2005;105(3):587‐92. [MEDLINE: ] - PubMed
Van Schoubroeck 2000 {published data only}
-
- Schoubroeck D, Verhaeghe J. Does local anaesthesia at mid‐trimester amniocentesis decrease pain experience? A randomised trial in 220 patients. Ultrasound in Obstetrics & Gynecology 2000;16:536‐8. - PubMed
Wax 2005 {published data only}
-
- Wax JR, Pinette MG, Carpenter M, Chard R, Blackstone J, Cartin A. A randomized single blinded trial of subfreezing versus room temperature needles to reduce pain with amniocentesis. Ultrasound in Obstetrics & Gynecology 2005;26:430. - PubMed
-
- Wax JR, Pinette MG, Carpenter M, Chard R, Blackstone J, Cartin A. Reducing pain with genetic amniocentesis ‐ a randomized trial of subfreezing versus room temperature needles. [Report No.] NAMC‐ACEL‐. United States. Air Crew Equipment Laboratory, Philadelphia 2005;18(4):221‐4. [MEDLINE: ] - PubMed
Zwinger 1994 {published data only}
-
- Zwinger A, Benešova O, Šmeral P, Mrazek M. The effectiveness of invasive procedures in prenatal diagnosis of genetic disorders. International Journal of Gynaecology and Obstetrics 1994;46:41.
Additional references
Agarwal 2012
-
- Mujezinovic F, Alfirevic Z. Procedure‐related complications of amniocentesis and chorionic villous sampling: a systematic review. Obstetrics and Gynecology 2007;110(3):687‐94. - PubMed
Akolekar 2015
-
- Akolekar R, Beta J, Picciarelli G, Ogilvie C, D’Antonio F. Procedure‐related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta‐analysis. Ultrasound in Obstetrics and Gynecology 2015;45:16‐26. - PubMed
Alfirevic 2002
Burton 1995
-
- Burton BK, Schultz CJ, Angle B, Burd LI. An increased incidence of haemangiomas in infants born following chorionic villus sampling (CVS). Prenatal Diagnosis 1995;15:209‐14. - PubMed
China 1975
-
- Department of Obstetrics and Gynecology, Tietung Hospital, Anshan, China. Fetal sex prediction by sex chromatin of chorionic cells during early pregnancy. Chinese Medical Journal 1975;1(2):117‐26. - PubMed
Froster 1996
-
- Froster UG, Jackson L. Limb defects and chorionic villus sampling: results from an international registry, 1992‐94. Lancet 1996;347:489‐94. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Jackson 1993
-
- Jackson L, Wapner RJ. Chorionic villus sampling. In: Simpson JL, Elias S editor(s). Essentials of prenatal diagnosis. New York: Churchill Livingstone, 1993:45‐62.
Mujezinovic 2007
-
- Mujezinovic F, Alfirevic Z. Procedure‐related complications of amniocentesis and chorionic villous sampling: a systematic review. Obstetrics and Gynecology 2007;110(3):687‐94. [MEDLINE: ] - PubMed
NICHHD 1993
-
- NICHHD. Report of the NICHHD workshop on chorionic villus sampling and limb and other defects. American Journal of Obstetrics and Gynecology 1993;169(1):1‐6. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 1988
-
- Robinson GE, Garner DM, Olmsted MP, Shime J, Hutton EM, Crawford BM. Anxiety reduction after chorionic villus sampling and genetic amniocentesis. American Journal of Obstetrics and Gynecology 1988;159:953‐6. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). The GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html Updated October 2013.
Spencer 1987
-
- Spencer JW, Cox DN. Emotional responses of pregnant women to chorionic villi sampling or amniocentesis. American Journal of Obstetrics and Gynecology 1987;157:1155‐60. - PubMed
Spencer 1988
-
- Spencer JW, Cox DN. A comparison of chorionic villi sampling and amniocentesis: acceptability of procedure and maternal attachment to pregnancy. Obstetrics and Gynecology 1988;72:714‐8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

