Cerebral near-infrared spectroscopy monitoring for prevention of brain injury in very preterm infants
- PMID: 28869278
- PMCID: PMC6483788
- DOI: 10.1002/14651858.CD011506.pub2
Cerebral near-infrared spectroscopy monitoring for prevention of brain injury in very preterm infants
Abstract
Background: Cerebral injury and long-term neurodevelopmental impairment is common in extremely preterm infants. Cerebral near-infrared spectroscopy (NIRS) enables continuous estimation of cerebral oxygenation. This diagnostic method coupled with appropriate interventions if NIRS is out of normal range (that is cerebral oxygenation within the 55% to 85% range) may offer benefits without causing more harms. Therefore, NIRS coupled with appropriate responses to abnormal findings on NIRS needs assessment in a systematic review of randomised clinical trials and quasi-randomised studies.
Objectives: To evaluate the benefits and harms of interventions that attempt to alter cerebral oxygenation guided by cerebral NIRS monitoring in order to prevent cerebral injury, improve neurological outcome, and increase survival in preterm infants born more than 8 weeks preterm.
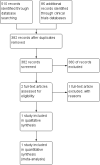
Search methods: We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 8), MEDLINE via PubMed (1966 to 10 September 2016), Embase (1980 to 10 September 2016), and CINAHL (1982 to 10 September 2016). We also searched clinical trial databases, conference proceedings, and the reference lists of retrieved articles for randomised clinical trials and quasi-randomised studies.
Selection criteria: Randomised clinical trials and quasi-randomised clinical studies comparing continuous cerebral NIRS monitoring for at least 24 hours versus blinded NIRS or versus no NIRS monitoring.
Data collection and analysis: Two review authors independently selected, assessed the quality of, and extracted data from the included trials and studies. If necessary, we contacted authors for further information. We conducted assessments of risks of bias; risks of design errors; and controlled the risks of random errors with Trial Sequential Analysis. We assessed the quality of the evidence with GRADE.
Main results: One randomised clinical trial met inclusion criteria, including infants born more than 12 weeks preterm. The trial employed adequate methodologies and was assessed at low risk of bias. One hundred and sixty-six infants were randomised to start continuous cerebral NIRS monitoring less than 3 hours after birth until 72 hours after birth plus appropriate interventions if NIRS was out of normal range according to a guideline versus conventional monitoring with blinded NIRS. There was no effect of NIRS plus guideline of mortality until term-equivalent age (RR 0.50, 95% CI 0.29 to 1.00; one trial; 166 participants). There were no effects of NIRS plus guideline on intraventricular haemorrhages: all grades (RR 0.93, 95% CI 0.65 to 1.34; one trial; 166 participants); grade III/IV (RR 0.57, 95% CI 0.25 to 1.31; one trial; 166 participants); and cystic periventricular leukomalacia (which did not occur in either group). Likewise, there was no effect of NIRS plus guideline on the occurrence of a patent ductus arteriosus (RR 1.96, 95% CI 0.94 to 4.08; one trial; 166 participants); chronic lung disease (RR 1.27, 95% CI 0.94 to 1.50; one trial; 166 participants); necrotising enterocolitis (RR 0.83, 95% CI 0.33 to 1.94; one trial; 166 participants); and retinopathy of prematurity (RR 1.64, 95% CI 0.75 to 3.00; one trial; 166 participants). There were no serious adverse events in any of the intervention groups. NIRS plus guideline caused more skin marks from the NIRS sensor in the control group than in the experimental group (unadjusted RR 0.31, 95% CI 0.10 to 0.92; one trial; 166 participants). There are no data regarding neurodevelopmental outcome, renal impairment or air leaks.The quality of evidence for all comparisons discussed above was assessed as very low apart from all-cause mortality and adverse events: these were assessed as low and moderate, respectively. The validity of all comparisons is hampered by a small sample of randomised infants, risk of bias due to lack of blinding, and indirectness of outcomes.
Authors' conclusions: The only eligible randomised clinical trial did not demonstrate any consistent effects of NIRS plus a guideline on the assessed clinical outcomes. The trial was, however, only powered to detect difference in cerebral oxygenation, not morbidities or mortality. Our systematic review did not reach sufficient power to prove or disprove effects on clinical outcomes. Further randomised clinical trials with low risks of bias and low risks of random errors are needed.
Conflict of interest statement
Gorm Greisen has done research using near‐infrared spectroscopy for 20 years and therefore could be perceived to be biased by academic interests.
Simon Hyttel‐Sørensen, Gorm Greisen, and Christian Gluud participated in the SafeBoosC trial (Hyttel‐Sorensen 2015a) which could bias their views.
Bodil Als‐Nielsen has none known bias risks.
Update of
- doi: 10.1002/14651858.CD011506
References
References to studies included in this review
Hyttel‐Sorensen 2015a {published data only (unpublished sought but not used)}
References to studies excluded from this review
Pichler 2016 {published data only}
-
- Pichler G, Urlesberger B, Baik N, Schwaberger B, Binder‐Heschl C, Avian A, et al. Cerebral oxygen saturation to guide oxygen delivery in preterm neonates for the immediate transition after birth: a 2‐center randomized controlled pilot feasibility trial. Journal of Pediatrics 2016;170:73‐8.e1‐4. [DOI: 10.1016/j.jpeds.2015.11.053; NCT02017691; PUBMED: 26743498] - DOI - PubMed
Additional references
Alderliesten 2014
Altman 2003
Angus 2015
-
- Angus DC. Fusing Randomized Trials With Big Data: The Key to Self‐learning Health Care Systems?. JAMA 2015;314(8):767‐68. - PubMed
Balegar 2014
Bell 1978
Benitz 2012
Boutron 2008
-
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P, CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Annals of Internal Medicine 2008;148(4):295‐309. [PUBMED: 18283207] - PubMed
Buunk 1998
-
- Buunk G, Hoeven JG, Meinders AE. A comparison of near‐infrared spectroscopy and jugular bulb oximetry in comatose patients resuscitated from a cardiac arrest. Anaesthesia 1998;53(1):13‐9. [PUBMED: 9505736] - PubMed
Casati 2005
-
- Casati A, Fanelli G, Pietropaoli P, Proietti R, Tufano R, Danelli G, et al. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesthesia and Analgesia 2005;101(3):740‐7. [DOI: 10.1213/01.ane.0000166974.96219.cd; PUBMED: 16115985] - DOI - PubMed
de Tournay‐Jetté 2011
-
- Tournay‐Jetté E, Dupuis G, Bherer L, Deschamps A, Cartier R, Denault A. The relationship between cerebral oxygen saturation changes and postoperative cognitive dysfunction in elderly patients after coronary artery bypass graft surgery. Journal of Cardiothoracic and Vascular Anesthesia 2011;25(1):95–104. [DOI: 10.1053/j.jvca.2010.03.019; PUBMED: 20650659 ] - DOI - PubMed
Denault 2007
Deschamps 2016
-
- Deschamps A, Hall R, Grocott H, Mazer CD, Choi PT, Turgeon AF, et al. Canadian Perioperative Anesthesia Clinical Trials Group. Cerebral oximetry monitoring to maintain normal cerebral oxygen saturation during high‐risk cardiac surgery: a randomized controlled feasibility trial. Anesthesiology 2016;124(4):826‐36. [DOI: 10.1097/ALN.0000000000001029; PUBMED: 26808629] - DOI - PubMed
Deulofeut 2006
Dullenkopf 2003
-
- Dullenkopf A, Frey B, Baenziger O, Gerber A, Weiss M. Measurement of cerebral oxygenation state in anaesthetized children using the INVOS 5100 cerebral oximeter. Pediatric Anesthesia 2003;13(5):384‐91. [PUBMED: 12791110] - PubMed
Finer 2010
-
- Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Early CPAP versus surfactant in extremely preterm infants. New England Journal of Medicine 2010;362(21):1970‐9. [DOI: 10.1056/NEJMoa091178; PUBMED: 20472939] - DOI - PMC - PubMed
GRADEpro GDT [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 17 April 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Greisen 2011
-
- Greisen G, Leung T, Wolf M. Has the time come to use near‐infrared spectroscopy as a routine clinical tool in preterm infants undergoing intensive care?. Philosophical Transactions. Series A, Mathematical, Physical, and Engineering Sciences 2011;369(1955):4440‐51. [DOI: 10.1098/rsta.2011.0261; PUBMED: 22006900] - DOI - PMC - PubMed
Herbert 2005
Heringlake 2010
Hessel 2014
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Highton 2010
-
- Highton D, Elwell C, Smith M. Noninvasive cerebral oximetry: is there light at the end of the tunnel?. Current Opinion in Anaesthesiology 2010;23(5):576‐81. [PUBMED: 20830845] - PubMed
Hirsch 2009
-
- Hirsch JC, Charpie JR, Ohye RG, Gurney JG. Near‐infrared spectroscopy: what we know and what we need to know ‐ a systematic review of the congenital heart disease literature. Journal of Thoracic and Cardiovascular Surgery 2009;137(1):154‐9. [DOI: 10.1016/j.jtcvs.2008.08.005; PUBMED: 19154918] - DOI - PubMed
Hou 2007
-
- Hou X, Ding H, Teng Y, Zhou C, Tang X, Li S, et al. Research on the relationship between brain anoxia at different regional oxygen saturations and brain damage using near‐infrared spectroscopy. Physiological Measurement 2007;28(10):1251–65. [DOI: 10.1088/0967-3334/28/10/010; PUBMED: 17906392] - DOI - PubMed
Hyttel‐Sorensen 2011
-
- Hyttel‐Sorensen S, Sorensen LC, Riera J, Greisen G. Tissue oximetry: a comparison of mean values of regional tissue saturation, reproducibility and dynamic range of four NIRS‐instruments on the human forearm. Biomedical Optics Express 2011;2(11):3047‐57. [DOI: 10.1364/BOE.2.003047; PUBMED: 22076266] - DOI - PMC - PubMed
Hyttel‐Sorensen 2013a
-
- Hyttel‐Sorensen S, Austin T, Bel F, Benders M, Claris O, Dempsey EM, et al. Clinical use of cerebral oximetry in extremely preterm infants is feasible. Danish Medical Journal 2013;60(1):A4533. - PubMed
Hyttel‐Sorensen 2013b
-
- Hyttel‐Sorensen S, Austin T, Bel F, Benders M, Claris O, Dempsey E, et al. A phase II randomized clinical trial on cerebral near‐infrared spectroscopy plus a treatment guideline versus treatment as usual for extremely preterm infants during the first three days of life (SafeBoosC): study protocol for a randomized controlled trial. Trials 2013;14:120. [DOI: 10.1186/1745-6215-14-120] - DOI - PMC - PubMed
Hyttel‐Sorensen 2015b
International Committee 2005
Ioannidis 2005
-
- Ioannidis JPA. Contradicted and initially stronger effects in highly cited clinical research. JAMA 205;294(2):218‐28. - PubMed
Ioannidis 2009
Jakobsen 2014
Jakobsen 2016
Khwaja 2008
Kuint 2009
Kurth 2002
Kurth 2009
Lemmers 2008
Lundh 2017
MacKay 2010
Meek 1999
Mohangoo 2011
-
- Mohangoo AD, Buitendijk SE, Szamotulska K, Chalmers J, Irgens LM, Bolumar F, et al. Euro‐Peristat Scientific Committee. Gestational age patterns of fetal and neonatal mortality in Europe: results from the Euro‐Peristat Project. PloS One 2011;6(11):e24727–12. [DOI: 10.1371/journal.pone.0024727; PUBMED: 22110575] - DOI - PMC - PubMed
Munro 2004
Murkin 2007
Nagdyman 2005
-
- Nagdyman N, Fleck T, Schubert S, Ewert P, Peters B, Lange PE, et al. Comparison between cerebral tissue oxygenation index measured by near‐infrared spectroscopy and venous jugular bulb saturation in children. Intensive Care Medicine 2005;31(6):846‐50. [DOI: 10.1007/s00134-005-2618-0; PUBMED: 15803294] - DOI - PubMed
Papile 1978
-
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] - PubMed
Pellicer 2013
-
- Pellicer A, Greisen G, Benders M, Claris O, Dempsey E, Fumagalli M, et al. The SafeBoosC Phase II randomised clinical trial: a treatment guideline for targeted near‐infrared‐derived cerebral tissue oxygenation versus standard treatment in extremely preterm infants. Neonatology 2013;104(3):171‐78. [DOI: 10.1159/000351346; PUBMED: 23921600] - DOI - PubMed
Perlman 2000
-
- Perlman JM, Rollins N. Surveillance protocol for the detection of intracranial abnormalities in premature neonates. Archives of Pediatrics & Adolescent Medicine 2000;154(8):822‐6. [PUBMED: 10922280] - PubMed
Petrova 2011
Plomgaard 2016
-
- Plomgaard AM, Oeveren W, Petersen TH, Alderliesten T, Austin T, Bel F, et al. The SafeBoosC II randomized trial: treatment guided by near‐infrared spectroscopy reduces cerebral hypoxia without changing early biomarkers of brain injury. Pediatric Research 2016;79(4):528‐35. [DOI: 10.1038/pr.2015.266; PUBMED: 26679155] - DOI - PMC - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivers 2001
-
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. The New England Journal of Medicine 2001;345(19):1368–77. - PubMed
Savović 2012a
-
- Savović J, Jones H, Altman D, Harris R, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1–82. [DOI: 10.3310/hta16350; PUBMED: 22989478] - DOI - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI: 10.7326/0003-4819-157-6-201209180-00537; DOI: ] - DOI - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Available from gdt.gradepro.org/app/handbook/handbook.html. Updated October 2013.
Slater 2009
Sorensen 2006
Stenson 2011
-
- Stenson B, Brocklehurst P, Tarnow‐Mordi W, U.K. BOOST II trial, Australian BOOST II trial, New Zealand BOOST II trial. Increased 36‐week survival with high oxygen saturation target in extremely preterm infants. New England Journal of Medicine 2011;364(17):1680‐2. [DOI: 10.1056/NEJMc1101319; PUBMED: 21524227] - DOI - PubMed
Takami 2010
Thorlund 2011
-
- Thorlund K, Engstrom J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). www.ctu.dk/tsa (accessed prior to 15 August 2017).
Toet 2006
Verhagen 2010
Volpe 2009
Watzman 2000
-
- Watzman HM, Kurth CD, Montenegro LM, Rome J, Steven JM, Nicolson SC. Arterial and venous contributions to near‐infrared cerebral oximetry. Anesthesiology 2000;93(4):947‐53. [PUBMED: 11020744] - PubMed
Wetterslev 2008
Wetterslev 2017
Whitelaw 2001
Wolf 2009
Wood 2008
-
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI: 10.1136/bmj.39465.451748.AD; PUBMED: 18316340] - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous