Review article: the human intestinal virome in health and disease
- PMID: 28869283
- PMCID: PMC5656937
- DOI: 10.1111/apt.14280
Review article: the human intestinal virome in health and disease
Abstract
Background: The human virome consists of animal-cell viruses causing transient infections, bacteriophage (phage) predators of bacteria and archaea, endogenous retroviruses and viruses causing persistent and latent infections. High-throughput, inexpensive, sensitive sequencing methods and metagenomics now make it possible to study the contribution dsDNA, ssDNA and RNA virus-like particles make to the human virome, and in particular the intestinal virome.
Aim: To review and evaluate the pioneering studies that have attempted to characterise the human virome and generated an increased interest in understanding how the intestinal virome might contribute to maintaining health, and the pathogenesis of chronic diseases.
Methods: Relevant virome-related articles were selected for review following extensive language- and date-unrestricted, electronic searches of the literature.
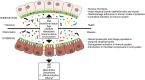
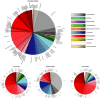
Results: The human intestinal virome is personalised and stable, and dominated by phages. It develops soon after birth in parallel with prokaryotic communities of the microbiota, becoming established during the first few years of life. By infecting specific populations of bacteria, phages can alter microbiota structure by killing host cells or altering their phenotype, enabling phages to contribute to maintaining intestinal homeostasis or microbial imbalance (dysbiosis), and the development of chronic infectious and autoimmune diseases including HIV infection and Crohn's disease, respectively.
Conclusions: Our understanding of the intestinal virome is fragmented and requires standardised methods for virus isolation and sequencing to provide a more complete picture of the virome, which is key to explaining the basis of virome-disease associations, and how enteric viruses can contribute to disease aetiologies and be rationalised as targets for interventions.
© 2017 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures
References
-
- Breitbart M, Rohwer F. Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 2005;13:278‐284. - PubMed
-
- Sullivan MB, Weitz JS, Wilhelm S. Viral ecology comes of age. Environ Microbiol Rep. 2017;9:33‐35. - PubMed
-
- Dalmasso M, Hill C, Ross RP. Exploiting gut bacteriophages for human health. Trends Microbiol. 2014;22:399‐405. - PubMed
-
- Keary R, Sanz‐Gaitero M, van Raaij MJ, et al. Characterization of a bacteriophage‐derived murein peptidase for elimination of antibiotic‐resistant Staphylococcus aureus . Curr Protein Pept Sci. 2016;17:183‐190. - PubMed
-
- Weinbauer MG. Ecology of prokaryotic viruses. FEMS Microbiol Rev. 2004;28:127‐181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
